
Dez. 3, 2024 - How Metaphors shape Biotechnology
Today, a book was published that is the result of a truly interdisciplinary collaboration between biotechnology and ethics at the Technical University of Braunschweig. It describes the influence of metaphors on life science research by integrateing systematic metaphor analysis with ethical, philosophical, and pedagogical perspectives on the significance of metaphors in biotechnology research and dissemination. Special attention was paid to metaphors’ role in public communication, potentially determining acceptance or rejection of biotechnological advances.
The book is available via open access: ISBN: 9783119145121

Nov. 25, 2025 - Visiting Uganda: TU scientists search for vaccine against East Coast fever
Professor Michael Hust and Biotechnologist Philip Heine visited Uganda. They worked together with Dr. Charles Ndawula and his team in the laboratory of the National Livestock Resources Research Institute (NaLIRRI). Their aim was the construction of gene libraries to identify biomarkers of the East Coast fever, which are an essential first step in the development of a vaccine against this disease that affects livestock such as cattle, goats, and sheep and causes a substantial economic damage in agriculture. The technology was developed at the TU Braunschweig. The collaboration between the two research institutions was further strengthened by a memorandum of understanding to promote student exchanges.
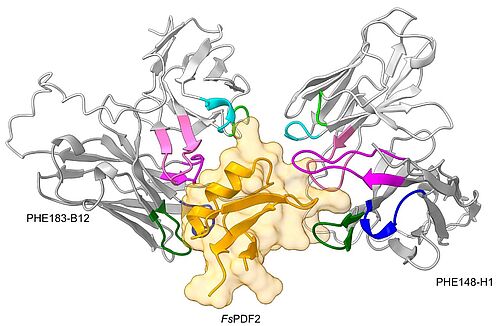
Nov 14, 2025 - New antibodies allow to monitor forest health
A team of scientists led by the Departments of Plant Biology and Biotechnology has identified a novel biomarker for forest health using animal-free antibody generation via phage display. They identified a novel antimicrobial peptide (defensin) in beech trees, a defence molecule involved in the response to biotic stress. Antibody-based tests that can detect these biomarkers directly in the forest will not only contribute to a better understanding of forest health, but also improve our understanding of plant immunity and the resilience and tolerance of trees to changing environmental influences.
Original publication: https://doi.org/10.1111/pbi.70431
Oct. 7, 2024 - Therapeutic antibodies against Marburg virus
Researchers at the Technical University of Braunschweig, together with partners in France and the USA, developed a therapeutic agent for the treatment of a Marburg virus infection. In a next step, an emergency drug could be produced under the quality conditions for therapeutics to treat the life-threatening infection.
more details: en / de

Sept. 20, 2024 - Award-winning German-Cuban cooperation in cancer therapy
Dr. Gertrudis Rojas Dorantes, a top scientist of the Cuban Centro de Inmunología Molecular (CIM), is visiting the Departments of Biotechnology and Medical Biotechnology at TUBS for the sixth time in 10 years. The international research collaboration developes improved therapeutic proteins for cancer therapy. The joint project was awarded with the Research Prize of the Cuban Academy of Sciences recently.
more details: en / de
Sept. 11, 2024 - Searching for a vaccine against East Coast fever
Ugandan guest scientist Dr Charles Ndawula from the National Livestock Resources Research Institute in Entebbe, Uganda, is spending two months at the Department of Medical Biotechnology. Here he will continue his research into the development of vaccines against East Coast Fever, a cattle disease, funded by a research fellowship in the prestigious ARISE programme of the African Academy of Sciences.
July 11, 2024 - Lecture for laypeople on the animal-free production of antibodies
In the framework of Phaeno Science Talks, Stefan Dübel explained the important role of antibodies for therapy, diagnostics and research, and how we can generate antibodies without the need to immunize animals.
Phaeno is a Science Museum in Wolfsburg with more than 350 interactive experiment stations for science and technology.
Video of the presentation (in german language)
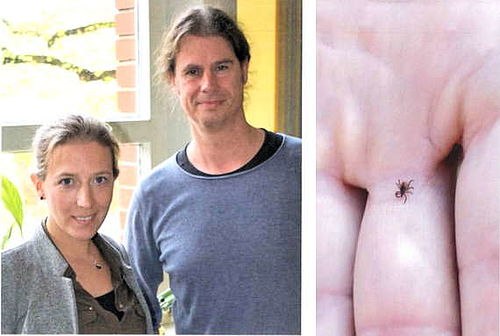
June 12, 2024 - Human antibodies against black widow toxin
Black widow bites can cause severe illness, which can be treated with horse-derived antibody sera. This method, however, can lead to severe side effects due to the animal origin. To change this, we have generated fully human antibodies that can neutralize the alpha-latrotoxin of the European black widow spider. These antibodies could provide an unlimited supply of a drug of constant quality and efficacy against latrodectism, but ore preclinical and clinical tests are needed before the antibodies can be used in practice.
more info in deutsch / english
press release of journal
original article (open access)

June 8, 2024 - The first generation of Braunschweig biotechnology doctoral students visit their alma mater after 50 years
Today, a group of former students of Prof. Fritz Wagner, founding Director of the Institute of Biochemistry and Biotechnology in 1972, visited the Biozentrum, where they worked on their doctoral theses. The current director, Prof. Stefan Dübel, welcomed the ten alumni, one of whom had even traveled all the way from New York, and presented the latest developments in antibody engineering, the institute's current research topic. After a tour of their old laboratories, the alumni continued their reunion in Braunschweig's restaurants and parks.
Apr. 18, 2024 - Can antibodies be vegan?
A guide to the production of antibodies: a new review leads through today's labyrinth of different antibody formats and the various methods of antibody production and their interrelationships. The evaluation of a large number of scientific studies also undeniably shows that antibodies can now be produced entirely without animal experiments for a large number of different applications. It therefore answers the question of whether antibodies can really be vegan.
Original publication (open access)

Feb.. 23, 2024 - Summer School in sustainable transformation
As part of an European Research Consortium, our spin off Startup ABCALIS is collaborating on the organization of an International Summer School about critical and interdisciplinary approaches to sustainable transformation. Named «Critical approaches to the sustainable transformation» the School will take place between July 15 and 19, 2024, in the University of Algarve (Gambelas Campus), in Faro, Portugal.
Deadline: April 15, 2024

Nov 24, 2023 - Biotechnology meets Philosophy
The truly interdisciplinary workshop "How Metaphors shape Biotechnology" brought together Biologists and Philosophers to discuss the impact of the metaphors on biosciences. Scientific research uses metaphors to guide the perception of research itself (epistemological capacity of metaphors) and to frame public science communication (discursive capacity). The use of Metaphors is essential to describe previously unknown biological phenomena and formulate hypotheses. The choice of a particular metaphor may well bias the researcher's thinking, and thus influence the direction of future reserach, since it always never exactly matches the true biological phenomenon. Metaphors are also essential to convey scientific results to a wider public, thus shaping the public perception and affect their acceptance or repudiation.

Nov 2, 2023 - Lehren aus der Pandemie – Medikamentenentwicklung im Fokus
Welche Lehren wir aus der Pandemie ziehen können und ob wir für die nächste Pandemie im Bereich Medikamentenforschung gut gewappnet sind - Antworten darauf gibt unser PaPräKa-Film. „PaPräKa“ steht für PandemiePräventionsKampagnen und ist ein Projekt der Metropolregion GmbH in Zusammenarbeit mit der Abteilung Biotechnologie der TU Braunschweig und dem Innovationszentrum Niedersachsen. PaPräKa unterstützt RAPID Niedersachsen (Response Against Pandemic Infectious Diseases), eine Initiative des Landes Niedersachsen für die Verbesserung der Reaktion auf zukünftige Pandemien. RAPID Niedersachsen zielt darauf ab, bei künftigen Pandemien eine effizientere Zusammenarbeit zwischen den betroffenen Akteur*innen in Wissenschaft, Wirtschaft und Politik zu ermöglichen und hat dazu einen konkreten Aktionsplan erarbeitet.
Sept 14, 2023 - RAPID conference identifies roadblocks for fast pandemic response
Stakeholders from various institutions met today in the Flife Science Factory in Göttingen to discuss how we can organise developments of medications against future pandemic threats more effective. At the conference organized by Papräka, the initiative "RAPID Niedersachsen" (Response against pandemic infectius diseases) presented a Workplan with 10 theses and detailed ideas how to improve the situation for reserach in future pandemics.
Download the RAPID Workplan (PDF)
Press information (in german)
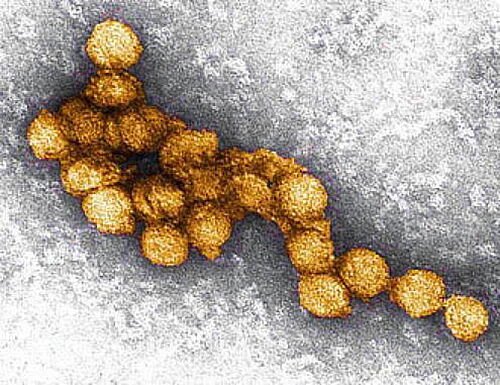
Sept 12, 2023 - Therapeutic antibodies and vaccines against West Nile virus
West Nile virus is transmitted by mosquitoes and is becoming increasingly prevalent in Europe, partly as a result of global warming. Infection with West Nile virus leads to encephalitis (inflammation of the brain) in about one in 100 infected persons, with about half of the patients suffering from late sequelae and cicra 10 percent of the patients die. So far, there is neither a specific therapy nor a vaccine against West Nile virus. Here, the TU Braunschweig will work together with partners in Spain, France and Denmark on new therapies and vaccines against this virus within the framework of an EU Horizon 2020 project.

Feb 8, 2023 - Vegan antibodies for alternatives to animal testing in research
The Department of Biotechnology will provide animal free „vegan“ antibodies for various projects within the research consortium "Micro-Replace Systems" that has received funding of 3.6 million euros from the Lower Saxony Ministry of Science and Culture (MWK) within the program "Zukunft.Niedersachsen". The consortium will develop chip-based replacement and supplementary methods to avoid animal testing. In 2017, the project started under the name "R2N - Replace and Reduce from Lower Saxony“.
Feb. 7, 2023 - Message from the Garage
The Braunschweig Startup Garage provides insights into motivation, success stories and challenges of company founders at first hand. This time, Dr. Al Halabi-Frenzel represents the Abcalis startup team of TU Braunschweig.
The Braunschweig Startup Garage is part of the Innovation Gateway of Braunschweig Stadtmarketing GmbH.
Feb. 5, 2023 - Abcalis wins W.IN
EXIST Forschungstransfer funded startup Team Abcalis at TU Braunschweig won additional support in the category "grow.in" of the W.IN academy of Braunschweig Zukunft GmbH. The startup academy W.IN provides mentors from the business community, networking and workshops with experienced entrepreneurs and case-related support on specific issues.
Start of W.IN (Braunschweiger Zeitung)
Dec. 8, 2022 - YUMAB celebrates 10th anniversary
With a ceremony attended by Lower Saxony's Minister for Science and Culture, Falko Mohrs, employees and friends of YUMAB celebrated the tenth anniversary of the company that brings recombinant human antibodies to the world. YUMAB was founded in December 2012 as a spin-off from TU Braunschweig by four scientists from the Institute of Biotechnology who had developed key technologies in phage display and antibody engineering.
In a lively podium discussion, the region's key players discussed the future of biotechnology in Lower Saxony and exchanged ideas on how to strengthen efforts to translate the often excellent scientific results of local academic institutes into practical applications and products.

Nov. 17, 2022 - Abcalis wins the Braunschweig Start-Up Award
The seventh Braunschweig Start-up Prize was awarded to TUBS-Spin-off company Abcalis by Braunschweig Zukunft GmbH and Braunschweigische Landessparkasse. The prize honors the innovative business ideas and courage of young companies from Braunschweig. The prize is endowed with a total of 10,000 euros. In addition, the Ströer company supports the winners with media services worth 15,000 euros.

15. Nov 2022 - Our former PhD student and now CEO of spin off Abcalis starrs on statewide startup campaign
Dr. Al-Halabi Frenzel, CEO of our spin off company Abcalis, starrs on more than 300 billboards throughout Lower Saxony as part of the campaign of the government to encourage students to think about starting a company.
Oct. 2022 - PaPräKa & RAPID: better Pandemic Prevention in the Metropolitan Region
The Video was shot at the one-day pandemic workshop on "RAPID: Response Against INfectious Pandemic Diseases" held as part of the PaPräKa project in Hanover.
More about RAPID Niedersachsen
More about Papräka

Oct 27, 2022 - Abcalis provides animal-free antibodies for semiconductor biosensors
The EU funded project “BIOASSEMBLER - Integrating bio-inspired assembly into semiconductor manufacturing technology for biosensors” produces a new generation of biosensors manufactured trough a bio-intelligent processes, marrying silicon with biomolecules. TUBS startup Abcalis provides the reagents for the the core of the detection process: innovatively engineered "vegan" antibody variants.


Sept 3, 2022: The Department of Biotechnology celebrates its 50th anniversary - a big party with visitors and Alumni from all around the world!
News entry of TU Magazine (in german)
Publication on the history of the Institute: Lang, S., Rau, U., Behrendt, C., Syldatk, C., Hust, M. & Dübel, S. (2022) Vom Gen zum Produkt. Nachr. Chemie 70, 7/8, 24-26
Aug 31, 2022 - Our Start-Up Abcalis wins High Tech Incubator funding.
Our Spin-off Abcalis is now part of the Göttingen Hightech Incubator Program, an initiative of the State of Lower Saxony to provide financial support for innovative startups. In a festive ceremony in Göttingen, Lower Saxony Minister for Economy, Labor, Transport and Digitalization, Dr. Bernd Althusmann announced the winners. CEO Laila Al-Halabi-Frenzel (left) and Head of Product Development, Dr. Esther Wenzel (right) represented Abcalis at the grant ceremony.
July 12, 2022 - CORAT Therapeutics wins 38.7 million of government funding
Our spin-off company CORAT Therapeutics GmbH has received approval for additional funding of up to € 38.7 Mio. from the "Clinical Development of Care-Related COVID-19 Drugs and Their Manufacturing Capabilities" program of the German Federal Ministry of Education and Research. This funding will support the clinical trials in up to 50 sites in six different countries, as well as the scaling and validation of the manufacturing process in preparation for market entry and supply.
press release (english) (german)
June 21, 2022 - Science Slam Winner
Dr. Kilian Zilkens, member of our Abcalis Startup Team, won this year's Science Slam at the Universities Festival of Sustainability TUmorrow Day 2022. A total of six slammers competed in the Science Slam. In the end, he convinced the audience in the Audimax with his lecture "Why Blind Detectives Should Be Vegan," in which he explained how we can save the lives of many laboratory animals by using our animal-free technologies to produce antibodies.

May 2022 - Abcalis selected as provider for the PETA Recombinant Antibody Challenge
PETA Science Consortium International e.V., the Physicians Committee for Responsible Medicine (PCRM), and the Alternatives Research and Development Foundation (ARDF) are offering grants for free catalogue recombinant antibodies for use in research and testing, where Researchers from any sector (e.g., academia, industry, or government) can apply to receive a recombinant antibody to test in their in vitro applications of interest.
Our Startup Abcalis is listed as one of the few eligible providers that meet the animal free production criteria of PETA Science Consortium International e.V.
May 17, 2022 - RAPID Workshop defines roadmap for better pandemics response
"Be fast. Have no regrets. You have to be the first mover. The Virus gets you if you do not move. If you have to be right before you move, you will never win. " With this quote from WHO's top pandemic manager Dr. Mike Ryan, Professor Melanie Brinkman, member of the German Federal Pandemics Expert Committe, emphasized the change in thinking that is necessary to save more lives in future pandemic situations. After inspiring welcome addresses from state secretaries Dr. Sabine Johannsen (Ministry of Science) and Stefan Muhle (Ministry of Economy), a top level team of stakeholders from scientific institutes and government agencies worked together to define the cornerstones of a roadmap of actions for an improved fast track development anti-viral drug developments in the future. Prof. Stefan Dübel, Initiator of RAPID, commented: "We are pleased to see the broad support and the many creative contributions from the attendands. If implemented, RAPID Niedersachsen will put us in a much better position to respond to the next pandemic much faster, better organised and more effectively."

March 30, 2022 - ABCALIS wins ECEAE Prize for animal-free antibodies
Our Abcalis Team was awarded the world's first award dedicated specifically to the development and application of non-animal derived antibodies. Co-Founder Dr. Esther Wenzel represented Abcalis at the award ceremony in Brussels. Non-animal derived antibodies do not need animal experiments for their generation and production, and provide always sequence defined detection reagents for reserach and diagnostics.
More info: ECEAE Award
We initiated the state initiative "RAPID Niedersachsen" with the goal to improve the development of anti-viral medications in future pandemics. The initiative will remove various "non-scientific" roadblocks and bottlenecks experienced in the development of anti-Corona medications, to speed up future pandemic responses.
Scientific coordination: Stefan Dübel & Maren Schubert
Project Manager: Allan Koch

2012 - 2022: TU Spinoff YUMAB celebrates its 10th anniversary!
Today, our Spin off YUMAB is a world leading provider of contract research, technologies, and R&D services for the development of fully human, therapeutic antibodies. See it's history here.

Braunschweig, February 1, 2022 - Dept. of Biotechnology joins COFONI.
The COVID-19 Research Network Lower Saxony (COFONI) is funding thirteen interdisciplinary collaborative projects in Lower Saxony with 5.97 million euros to find new medications against SARS-CoV-2 and to investigate the causes and long-term consequences of a COVID-19 disease. In this renewed project funding round, COFONI has now included the Biotechnology Department of the Technical University of Braunschweig as a new research partner. We are working on better prediction of future variants of SARS-CoV-2 and improved medications against it (project PREMUS, Prediction of Escape Mutants).
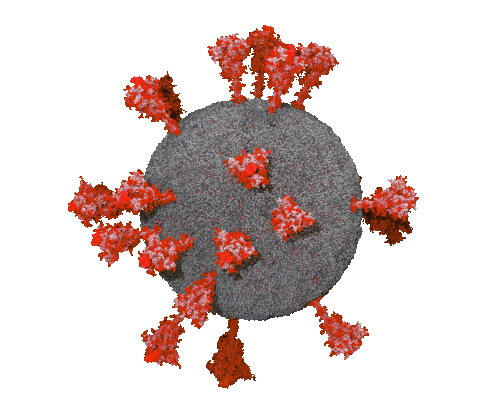
Dec 17, 2021 - ABCALIS diagnostic/research antibody against anti-SARS-CoV-2 Spike Protein S1 (RBD) binds to Omikron
The phage display derived antibody AB68-A09 made by Abcalis shows the same detection sensitivity for Wuhan, Delta and Omikron variants of the spike protein. The antibody was generated with animal free methods (i.e. without an immunisation of an experimental animal, and with production methods that do not use animal materials) by recombinant means and detects the receptor binding domain of the COVID-19 causing virus SARS-CoV-2.
The antibody is available here
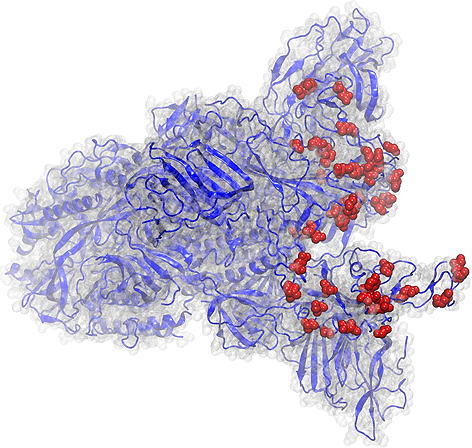
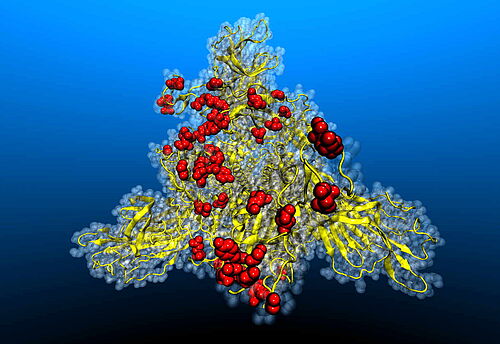
Braunschweig , Dec 10, 2021 / Jan 11, 2022 - Boost immunizations help against Omicron
After vaccination against the coronavirus, our body produces antibodies that contribute significantly to protection against COVID-19. The amount and strength of binding of these antibodies to the new omicron variant and the efficiency of different vaccination combinations have been investigated in two different studies by a team of researchers from the Technical University of Braunschweig together with partners from the EU consortium ATAC (Antibody Therapy Against Corona), as well as the Helmholtz Centre for Infection Research and the University of Rijeka. The results contribute to a better understanding of the spreading properties of the virus, and show that boost immunization increases the number of antibodies against the envelope protein of SARS-CoV-2 in all virus variants studied, including the new omicron variant. Important findings also relate to boost immunization following dual vaccination with the Sinovac or Sinopharm inactivated vaccines commonly used outside of Europe.
An important and somehow surprising finding of the first study is that the Omicron variant of the spike protein bound the cell surface receptor less efficiently than the currently circulating virus (see figure). On the other hand, both COVID patients and vaccinated persons have reduced antibody responses against Omicron. This suggests that 15 mutations present in the Omicron RBD are there rather to allow the virus to escape recognition but not to infect cells more efficiently.
Press release (engl.) / Pressemeldung (deutsch) on virus infectivity
Original publickation: BMC Medicine 20:102

Braunschweig, Jan. 11, 2022 - Worldwide study on efficacy of cross-vaccinations
In a second study, serum samples from COVID-19 convalescents and people immunized with mRNA vaccines (BioNTech, Moderna) or inactivated vaccines (Sinovac, Sinopharm) were examined. It was shown that the amount of antibodies in the blood against the envelope protein of SARS-CoV-2 started to decrease about three months after the second vaccination. However, boost immunization again increased the amount of specific antibodies, even to higher levels than immediately after the second vaccination. This could be shown for all four virus variants of concern (VOC): the original Wuhan variant, as well as Beta, Delta and Omicron. For the Wuhan variant, the number of immune cells (B and T lymphocytes) reacting against the virus was also determined. Here again, boost immunization resulted in an impressively improved immune response. The highest antibody levels were found in triple mRNA vaccinated and – most important – also in those vaccinated first with an “inactivated vaccine” and then boosted with an mRNA vaccine.
Press release (engl.) / Pressemeldung (deutsch) on cross vaccination efficiency
Original Study on cross vaccination efficiency
(the manuscripts are not yet peer-reviewed and should not be relied upon without context to guide clinical praxis or health related behaviour and should not be reported in news media as established information without consulting multiple experts in the field)

Braunschweig, Sept 6, 2021 - Substantial support for CORAT Therapeutics by the German federal government
Federal Secretary of Research Anja Karliczek and Federal Secretary of Health Jens Spahn announced today the funding of CORAT through the programme "Clinical Development of Care-Related COVID-19 Drugs and Their Manufacturing Capacities" by BMG and BMBF.
Federal Research Minister Anja Karliczek explains: "For several weeks now, the delta variant of the SARS-CoV-2 virus has clearly shown us that we will unfortunately have to expect that people will continue to fall ill with COVID-19 in the future. Even with widespread availability of COVID-19 vaccines, not all people will be able to receive vaccination. Therefore, more effective drugs are needed to treat the ill."
Federal Secretary of Health Jens Spahn stated:
"At the moment, only vaccination against Corona helps. But we also want to take the horror out of the pandemic in the long term. That's why we are supporting the development of new drugs so that we can continue to treat Corona patients well in the future. And we are investing in the future because in doing so we are also strengthening Germany as a biotechnology research and development location."
Press Release No. 179/2021 from BMG and BMBF (PDF)
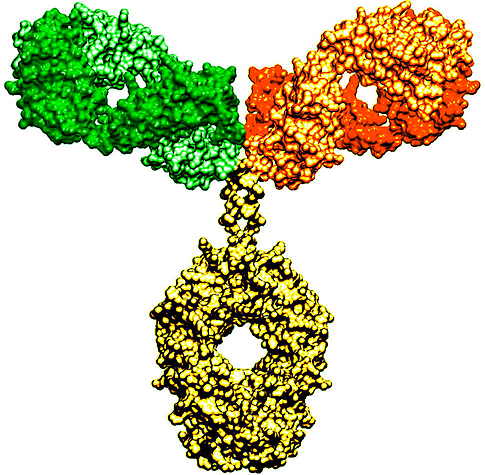
Braunschweig, Germany, June 22, 2021 - CORAT antibody COR-101 binds and inhibits all B.1.617 SARS-CoV-2 variants, including the fast proliferating “delta” variant
The newest laboratory tests indicate that COR-101 binds and inhibits the particularly aggressive new „delta” variant B.1.617.2, which is rapidly replacing the “alpha” variant B.1.1.7. On May 10th, the WHO classified the B.1.617.2 SARS-CoV-2 variant, first detected in India, as “of concern”. According to current data from the European Centre for Disease Prevention and Control (ECDC) the “delta” variant B.1.617.2 has replaced previous SARS-CoV-2 variants, including the “alpha” variant B.1.1.7, currently prevalent in Germany. According to ECDC analysis, the mutations likely increase transmissibility and decrease neutralization by convalescent plasma and currently approved therapeutic antibodies, explaining the sharp increase in the number of cases in India in recent weeks. The currently used vaccines obviously have different efficacies against the newly emerging variants. Also, while vaccines can protect healthy people, they cannot cure people already suffering from COVID-19; moreover, not everyone responds to vaccination. For these patient groups, CORAT Therapeutics GmbH is developing a drug that has an effect against SARS-CoV-2 directly.

Braunschweig, June 03, 2021 - CORAT secures 12,7 Mio € from Lower Saxony State, private investors, and the German Ministry of Research and Education (BMBF)
NBank Capital and private investors from Braunschweig, each having participated in previous financing rounds, renewed their investments. The current round of financing includes additional new investors from Braunschweig. Additionally, CORAT Therapeutics GmbH is to receive up to seven million euros from the federal funding program „Research and development of urgently needed therapeutics against SARS-CoV-2“ to support clinical development of COR-101. The goal of the current financing round is to secure funding for a Phase II clinical trial with the antibody drug candidate COR-101.
Lower Saxony State Secretary of Economics, Dr. Bernd Althusmann, comments:
"CORAT has completed all the necessary development steps on schedule so far. Now, it is important to maintain the fast development and to continue with the necessary clinical trials without delay. The government of Lower Saxony is excited to continue this success story."
4/23/2021 - First COVID-19 patients treated with COR-101 drug developed in Braunschweig, Germany.
Effective drugs against COVID-19 remain scarce in the current coronavirus pandemic. Even though the focus for months has been primarily on enabling rapid vaccination, there will be many people in the future who cannot be vaccinated, for example, due to concomitant diseases, or in whom the vaccination effect fails. Therefore, it is essential to quickly develop specific drugs for the treatment of COVID-19 disease.
The Corona Antibody Team (CORAT) of TU Braunschweig, led by Prof. Michael Hust and Prof. Stefan Dübel, and the biotechnology company YUMAB GmbH in Braunschweig have developed the virus-neutralizing antibody COR-101, which is highly effective against SARS-CoV-2 in laboratory tests. For the clinical development of COR-101, CORAT Therapeutics GmbH was founded with the support of the state of Lower Saxony, which has now started the first clinical trials in collaboration with t the University Hospital of Tübingen. Further clinical study centers are located in Braunschweig, Stuttgart (Robert Bosch Hospital), Leipzig and Dresden. The Phase Ib/II study is being conducted at these sites. In Phase-I, the tolerability of the antibody is initially being investigated in four dose cohorts. Phase II results on efficacy may form the basis for a marketing authorization application for the drug. Initial interim results are expected in the summer.
The antibody COR-101 differs in one important respect from the antibody preparations previously approved in the USA, which were procured by the German Federal Ministry of Health: COR-101 is specifically designed to treat hospitalized moderately to severely ill patients, for whom the U.S. antibodies must not be used because of their side effects. COR-101 avoids these side effects through a different, specially adapted molecular design.
Dr. Bernd Althusmann, Lower Saxony Minister of Economic Affairs, comments: "The promising antibody drug COR-101 shows that research made in Lower Saxony can provide an important building block on the way out of the pandemic. The clinical test phase that started yesterday gives hope that the Braunschweig project can soon save lives."
Further details:
press release of CORAT Therapeutics
Website of CORAT Therapeutics
more about the Mechanism of action of COR-101 here (animation) and here (crystal structure)

31.3.2021 - Farewell to Prof. Dr. Udo Rau
After more than 40 years at the Department of Biotechnology, Prof. Rau is released to his well-deserved retirement with a corona-style handshake from Prof. Stefan Dübel. Prof. Rau already earned his doctoral degree in the Biotechnology Departent in 1984, gained his Habilitation in the 1990es and served for many years as Dean of Biotechnology Studies. Udo, you will be missed!
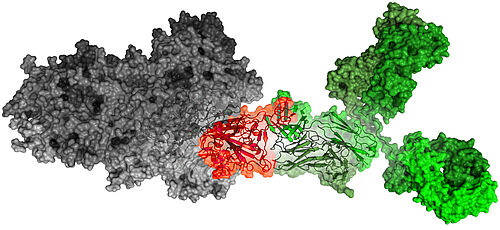
March 29, 2021 - Braunschweig antibody in clinical trials in the USA: New Drug Grips Tumor Cells With Two Arms.
The first patients with advanced tumors have been treated with a novel antibody-based in a clinical trial in the USA. It uses an invention of the Technical University of Braunschweig: The team of Professor Stefan Dübel, head of the Department of Biotechnology, discovered a human antibody that targets tumor cells and developed it over many years. This antibody is now part of a complex novel compound against difficult-to-treat cancers that is undergoing clinical testing by the pharmaceutical company Merck.
March 16, 2021 - CORAT Therapeutics GmbH obtained regulatory authorization for clinical phase I/Ib trial with the SARS-CoV-2 neutralizing human antibody COR-101.
The study (ClinicalTrials.gov ID: NCT04674566) is a randomized, double-blind, placebo-controlled, parallel-group, multicenter, first-in-human, phase I/Ib study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, immunogenicity, and efficacy of COR-101 in hospitalized patients with moderate to severe COVID-19.
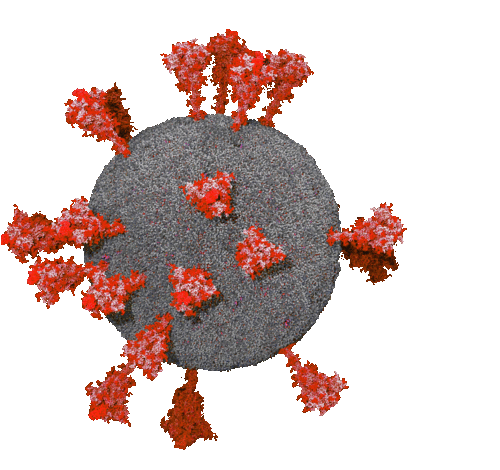
March 11, 2021 - Neutralizing anti-SARS-CoV-2 antibodies from immune cells of healthy donors
An international research team of the Technische Universität Braunschweig and the Helmholtz Centre for Infection Research (HZI), led by the Department of Biotechnology of the TU Braunschweig, reported the development of virus-neutralizing antibodies against SARS-CoV-2 in the journal Nature Communications. These antibodies prevent the viruses from entering host cells and were isolated from an antibody gene library that had already been produced from immune cells of healthy donors before the pandemic. The study shows how, in the future, active substances to previously unknown, emerging viruses can be produced very quickly without the need for patient-derived material.
original publication (open access)

3/2021 - The team of the Department of Biotechnology led by Dr. Maren Schubert won the second prize in the CHALLENE ONE HEALTH HACKATHON for their project "PANDAAlarmRed", an early warning structure against future emerging zoonotic virus diseases than may cause the next pandemics.
18. Februar 2021 - A team of researchers from the Technische Universität Braunschweig, together with partners from the EU consortium ATAC (Antibody Therapy Against Corona), has published the results of studies on the longevity of the immune response of COVID-19 patients in the medical journal "Med" published by Cell Press.

12/2020 - In an event with Economics Minister Dr. Althusmann, Dr. Sabine Johannsen, State Secretary for Science and Culture and Stefan Muhle, State Secretary in Lower Saxony’s Ministry of Economics, Labour, Transport and Digitisation, three of our startups won in three of the four categories of the Durchstarterpreis 2020:
December 18, 2020 - Human SARS-CoV-2 neutralizing antibody COR-101 demonstrates high efficacy in a COVID-19 animal model and is ready to be used in clinical studies to treat COVID-19 patients
CORAT Therapeutics GmbH today reports the successful conclusion of hamster disease model tests of their lead candidate COR-101 against COVID-19. "Our antibody induced recovery of SARS-CoV-2 infected hamsters within two days. In contrast, without treatment, the hamsters only started to reach the same health status after a full week." comments Dr. Andreas Herrmann, CEO of CORAT Therapeutics.
Treatment with COR-101 substantially reduced the weight loss after infection, which indicates the general health state. But since the main life-threatening effects of the virus are happening in the lung, the virus titer was measured directly in this organ which mainly contributes to lethality of SARS-CoV-2.
Dr. Herrmann says: "We are especially happy to report that COR-101 drastically reduced the amount of SARS-CoV-2 in the lung. Already after three days, an average of less than 1 % of the virus titer of the untreated control group could be detected after treatment with COR-101. This confirms the excellent neutralizing capability that we already measured in the laboratory. It demonstrates that COR-101 efficiently neutralizes the virus in a living organism and prevents disease progression".
In that respect, Dr. Herrmann also emphasized the difference in effector mechanisms compared to other antibodies: "In contrast to plasma therapy and the currently emergency approved antibodies in the US, our recombinant antibody is especially designed not to induce overshooting immune responses which contribute to the lethality in the lung. We achieved this by carefully removing the respective signals from the molecule before starting its production."
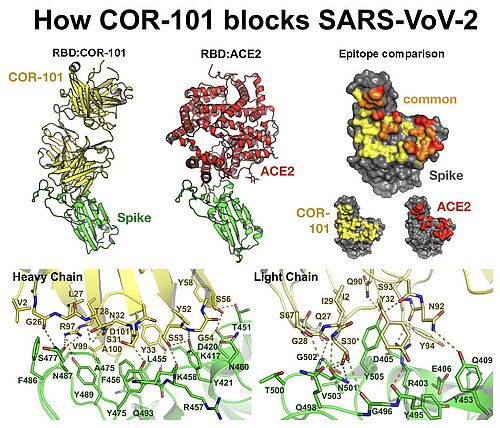
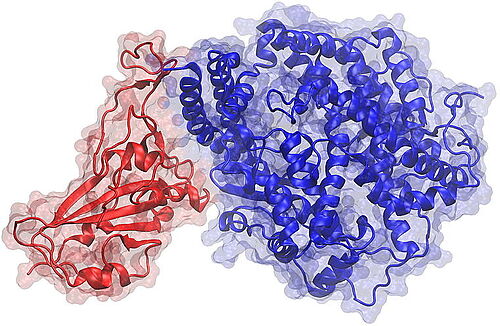
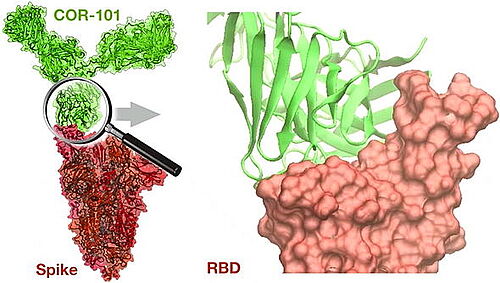
4.12.2020 - CORAT today published how COR-101 blocks SARS-CoV-2 and prevents infection with the coronavirus. The fully human antibody COR-101 showed an IC50 of 0.56 nM in a plaque-based live SARS-CoV-2 neutralization assay. The crystal structure of COR-101 in complex with SARS-CoV-2-RBD was solved at 2.0 A resolution showing that the antibody binds at the same region as the human coronavirus receptor ACE2 to SARS-CoV-2-RBD. In contrast to other published anti-SARS-CoV-2 antibodies, the binding of STE90-C11 is not blocked by known virus mutations, endowing COR-101 with higher intrinsic resistance to those possible escape mutants.
CORAT CEO Dr. Andreas Herrmann explains: "We know that a vaccine does not work in every person, and this is especially observed in older people. There are patients with other diseases that cannot be vaccinated as well. Unfortunately, these are precisely the two groups of people who usually have a higher risk of developing COVID-19. This is where COR-101 should help. Since COR-101 permanently occupies the essential contact point between the virus and our body, the virus can no longer use its "spike" proteins to infect us. We therefore expect COR-101 to be able to help all those who already have COVID-19 but whose immune system was unable to initiate its own antibody response."
Unlike the typical vaccines currently under investigation, antibody drugs do not require sophisticated freezing logistics, they are very stable and robust molecules. A century ago, doctors carried vials of antibodies in their pockets for weeks without refrigeration, so that they were always ready for use against tetanus or diphtheria, for example. COR-101 is therefore expected to have much simpler and cheaper logistics for the distribution than RNA vaccines.

18.11.2020 - CORAT lead product COR-101 ready for clinical trials
CORAT Therapeutics CEO Dr. Andreas Herrmann today reports the conclusion of the production campaign providing the first larger batch of COR-101 ready for the patients: „We are extremely happy and would like to thank everybody who helped to make this happen so quickly. With acute case numbers rising fast, CORAT hopes to make a difference by providing an option soon to directly treat the Coronavirus-Infected. We hope that the clinical studies now can commence very soon.“

9/2020 - Double success! From 120 applicants, our teams were selected to win two of the three Lower Saxony Innovation Awards for 2020:

CORAT is one of the winners at 2020 FallingWalls Science Conference in Berlin

16.6.2020 - With support from the German Federal State of Lower Saxony, CORAT Therpeutics was formed as a dedicated body to promote the further development of the antibody based COVID-19 medicine.
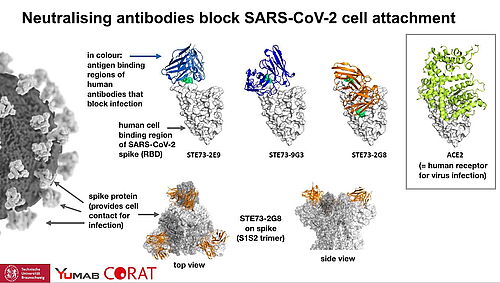
5.6.2020 - CORAT publishes the first substantial dataset describing fully human antibodies from heathly donors that are able to block the infection by SARS-CoV-2.

5/2020 A new EU commission white paper on animal use for antibody generation prominently acknowledges the animal-free multiclonal antibodies invented by the TU Braunschweig spinoff/EXIST founder team ABCALIS.
Summary in Nature
Independent comment of the experts in mabs

4/2020: Helping to fight against COVID19:
CORAT member YUMAB, spinoff of the Department of Biotechnology of the TU Braunschweig, generated a neutralising coronavirus antibody protecting against SARS-CoV-2 infection, as confirmed by the Helmholtz Center for Infection Reserach

2020: Learning close to practical applications: For the first time, Fraunhofer ITEM and TU Braunschweig held a joint internship on the topic of "Antibody drugs: from gene to product". Read more in the TU Braunschweig magazine.
2020: "Founder of the month" - featured by the magazine "Wirtschaft"

2019: Innovation Prize of the German Bioregions was awarded to our Norden Vaccines Team at the German Biotechnology Days in Wuerzburg, April 9th

2018: Biotechnology student excursion "Running Biotechnology with low Resources" to Universiti Sains Malaysia (USM). A report is available in the TU Braunschweig magazine.

2018: Winners of the GO-Bio competition of the German Ministry for Education and Research for a project to develop a novel type of vaccine against several different tick-borne diseases (e.g. Borreliosis)

2017: 1st prize of the Innovationsnetzwerk Niedersachsen was awarded to Yumab and the TU Braunschweig at the Hannover Messe by the Minister for Science and Culture, Gabriele Heinen-Kljajić, and the Minister for Economics, Employment and Traffic, Olaf Lies

2016: Technology Transfer Award of the Chamber of Commerce Braunschweig
Four scientists of the Department of Biotechnology, Dr. Andre Frenzel, Dr. Thomas Schirrmann, Prof. Michael Hust and Prof. Stefan Dübel, were awarded for creating the University spin-off company Yumab GmbH
.

2015: Innovation in Biotechnology Award of the American Association of Pharmaceutical Scientists (AAPS) for Prof. Stefan Dübel
2014: Science Award of the State of Lower Saxony for our two PhD students Tobias Unkauf and Jonas Zantow (picture: with the Lower Saxony Minister for Science and Culture, Gabriele Heinen-Kljajić).

2014: Gold medal and Lehr-LEO: Award for good teaching from TUBS for our iGEM project and a Gold Medal in Boston for the team of Braunschweig students for their project to fight the world climate change.
iGEM WIKI with the details of the project

2013: World champion "Best New Application": Our TU Braunschweig team won in the iGEM competition in synthetic biology at the MIT in Boston with work on the biotechnological application of bacterial quorum sensing.
iGEM Wiki with all the details on the project
21.11.2013 - Prof. Stefan Dübel receives the Entrepreneurship Award
For the first time, Ostfalia and the TU Braunschweig have presented the "Entrepreneurship Award" for special commitment to the establishment of a lively start-up culture. Prof. Dr. Rosemarie Karger, designated president of Ostfalia, and Prof. Dr. Ulrich Reimers, Vice President of the TU Braunschweig, presented the award in the Council Hall of the City of Wolfenbüttel.

25.2.2011 - Farewell to Prof. Dr. Siegmund Lang
Prof. Lang, one of the first members of the Department of Biotechnology, is honoured by a ceremony in presence of many of his colleagues and friends. Prof. Lang already earned his doctoral degree in the Biotechnology Departent in 1975, gained his Habilitation in the 1990es and served for many years as Dean of Biotechnology Studies. Sigi, we will miss you!
