⚈ News ⚈ What we do ⚈ How it works ⚈ What's the Difference to Vaccines ⚈ Technology ⚈ Team ⚈ Antibodies for Diagnostics ⚈ References
July 12, 2022 - CORAT Therapeutics wins 38.7 million of government funding
Our spin-off company CORAT Therapeutics GmbH has received approval for additional funding of up to € 38.7 Mio. from the "Clinical Development of Care-Related COVID-19 Drugs and Their Manufacturing Capabilities" program of the German Federal Ministry of Education and Research. This funding will support the clinical trials in up to 50 sites in six different countries, as well as the scaling and validation of the manufacturing process in preparation for market entry and supply.
press release (english) (german)

We initiated the state initiative "RAPID Niedersachsen" with the goal to improve the development of anti-viral medications in future pandemics. The initiative will remove various "non-scientific" roadblocks and bottlenecks experienced in the development of anti-Corona medications, to speed up future pandemic responses.
Coordinator: Allan Koch
Scientific coordination: Stefan Dübel & Maren Schubert
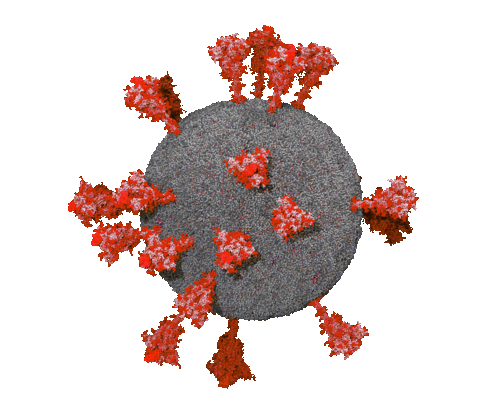
Braunschweig , Dec 10, 2021 / Jan 11, 2022 - Boost immunizations help against Omicron
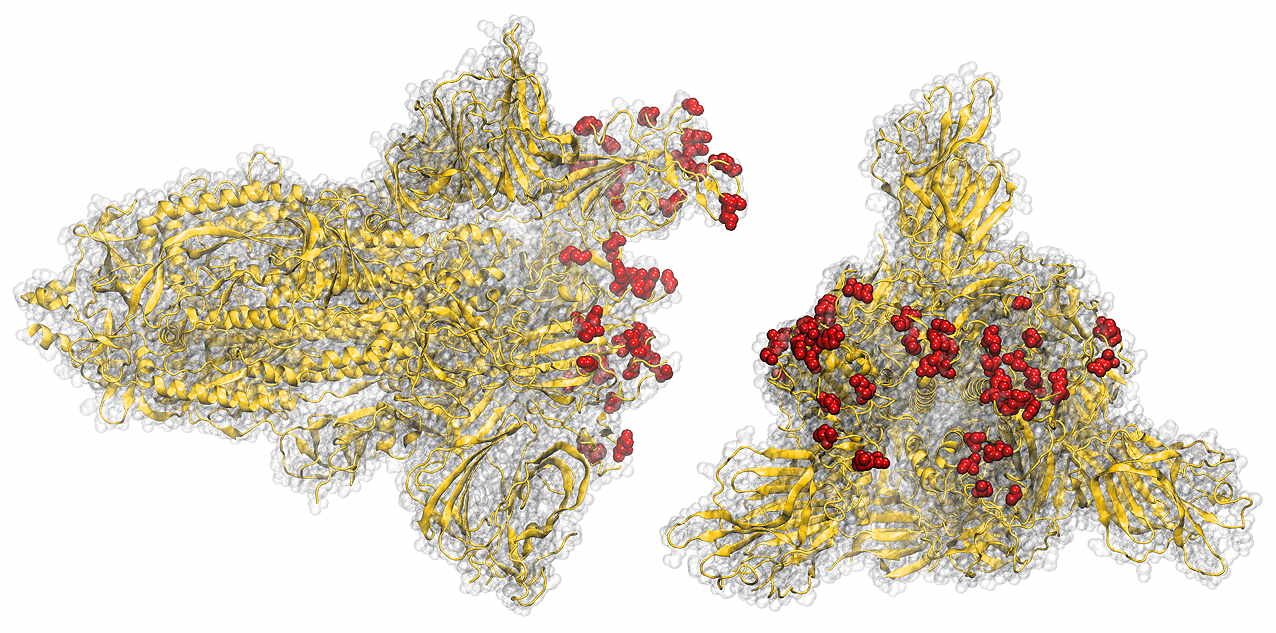
After vaccination against the coronavirus, our body produces antibodies that contribute significantly to protection against COVID-19. The amount and strength of binding of these antibodies to the new omicron variant and the efficiency of different vaccination combinations have been investigated in two different studies by a team of researchers from the Technical University of Braunschweig together with partners from the EU consortium ATAC (Antibody Therapy Against Corona), as well as the Helmholtz Centre for Infection Research and the University of Rijeka. The results contribute to a better understanding of the spreading properties of the virus, and show that boost immunization increases the number of antibodies against the envelope protein of SARS-CoV-2 in all virus variants studied, including the new omicron variant. Important findings also relate to boost immunization following dual vaccination with the Sinovac or Sinopharm inactivated vaccines commonly used outside of Europe.
An important and somehow surprising finding of the first study is that the Omicron variant of the spike protein bound the cell surface receptor less efficiently than the currently circulating virus (see figure). On the other hand, both COVID patients and vaccinated persons have reduced antibody responses against Omicron. This suggests that 15 mutations present in the Omicron RBD are there rather to allow the virus to escape recognition but not to infect cells more efficiently.
Worldwide study on efficacy of cross-vaccinations
In a second study, serum samples from COVID-19 convalescents and people immunized with mRNA vaccines (BioNTech, Moderna) or inactivated vaccines (Sinovac, Sinopharm) were examined. It was shown that the amount of antibodies in the blood against the envelope protein of SARS-CoV-2 started to decrease about three months after the second vaccination. However, boost immunization again increased the amount of specific antibodies, even to higher levels than immediately after the second vaccination. This could be shown for all four virus variants of concern (VOC): the original Wuhan variant, as well as Beta, Delta and Omicron. For the Wuhan variant, the number of immune cells (B and T lymphocytes) reacting against the virus was also determined. Here again, boost immunization resulted in an impressively improved immune response. The highest antibody levels were found in triple mRNA vaccinated and – most important – also in those vaccinated first with an “inactivated vaccine” and then boosted with an mRNA vaccine.
Press release (engl.) / Pressemeldung (deutsch) on virus infectivity
Press release (engl.) / Pressemeldung (deutsch) on cross vaccination efficiency
Original Study on virus infectivity
Original Study on cross vaccination efficiency
(the manuscripts are not yet peer-reviewed and should not be relied upon without context to guide clinical praxis or health related behaviour and should not be reported in news media as established information without consulting multiple experts in the field)

CORAT has developed an immunotherapy against COVID-19 using fully human monoclonal antibodies to prevent SARS-CoV2 infection.
Academic Initiators: Prof. Dr. Stefan Dübel, Prof. Dr. Michael Hust (TU Braunschweig).
Director, CORAT Therapeutics GmbH: Dr. Andreas Herrmann
CORAT Therapeutics GmbH now coordinates the clinical development
Braunschweig, February 1, 2022 - Dept. of Biotechnology joins COFONI.
The COVID-19 Research Network Lower Saxony (COFONI) is funding thirteen interdisciplinary collaborative projects in Lower Saxony with 5.97 million euros to find new medications against SARS-CoV-2 and to investigate the causes and long-term consequences of a COVID-19 disease. In this renewed project funding round, COFONI has now included the Biotechnology Department of the Technical University of Braunschweig as a new research partner. We are working on better prediction of future variants of SARS-CoV-2 and improved medications against it (project PREMUS, Prediction of Escape Mutants).
Braunschweig, Dec 17, 2021 - Diagnostic/research antibody against anti-SARS-CoV-2 Spike Protein S1 (RBD) binds to Omikron
The phage display derived antibody AB68-A09 made by our Abcalis Startup Team shows the same detection sensitivity for Wuhan, Delta and Omikron variants of the spike protein. The antibody was generated with animal free methods (i.e. without an immunisation of an experimental animal, and with production methods that do not use animal materials) by recombinant means and detects the receptor binding domain of the COVID-19 causing virus SARS-CoV-2. The antibody is available here
Braunschweig, Sept 6, 2021 - Substantial support for CORAT Therapeutics by the German federal government
Federal Secretary of Research Anja Karliczek and Federal Secretary of Health Jens Spahn announced today the funding of CORAT through the programme "Clinical Development of Care-Related COVID-19 Drugs and Their Manufacturing Capacities" by BMG and BMBF.
Federal Research Minister Anja Karliczek explains: "For several weeks now, the delta variant of the SARS-CoV-2 virus has clearly shown us that we will unfortunately have to expect that people will continue to fall ill with COVID-19 in the future. Even with widespread availability of COVID-19 vaccines, not all people will be able to receive vaccination. Therefore, more effective drugs are needed to treat the ill."
This renewed support for the clinical development of our antibody COR101 aims to ensure that candidates for new drugs that have been successfully tested in clinical phases I and II reach patients in Germany as quickly as possible. This is intended to promptly expand the options for treating COVID-19 in the long term in line with patient needs.
Federal Secretary of Health Jens Spahn stated:
"At the moment, only vaccination against Corona helps. But we also want to take the horror out of the pandemic in the long term. That's why we are supporting the development of new drugs so that we can continue to treat Corona patients well in the future. And we are investing in the future because in doing so we are also strengthening Germany as a biotechnology research and development location."
Press Release No. 179/2021 from BMG and BMBF (PDF)
Braunschweig, July 7, 2021 - CORAT Therapeutics GmbH announces partnership with Dermapharm Holding SE as strong strategic partner for the further development of the antibody COR-101 discovered at TU Braunschweig
For the fast and efficient delivery of its innovative drug COR 101 against COVID-19, CORAT Therapeutics GmbH announces the partnership with Dermapharm Holding SE, a manufacturer of branded pharmaceuticals. The partnership is intended to accelerate clinical development and facilitate the expansion of COR-101 production to make the drug available to patients more quickly.
"Currently, there is no approved, effective, antiviral-specific treatment for hospitalised patients with moderate to severe COVID-19 symptoms. While vaccines will remain the most important tool in fighting the pandemic, they will not be able to completely prevent the occurrence of severe cases of the disease. Therefore, this therapy is another tool that is needed. By acquiring the equity investment in CORAT we are not only providing the funds needed to accelerate the development process, but also our expertise in manufacturing antibody drugs," said Dr Hans-Georg Feldmeier, CEO of Dermapharm Holding SE.
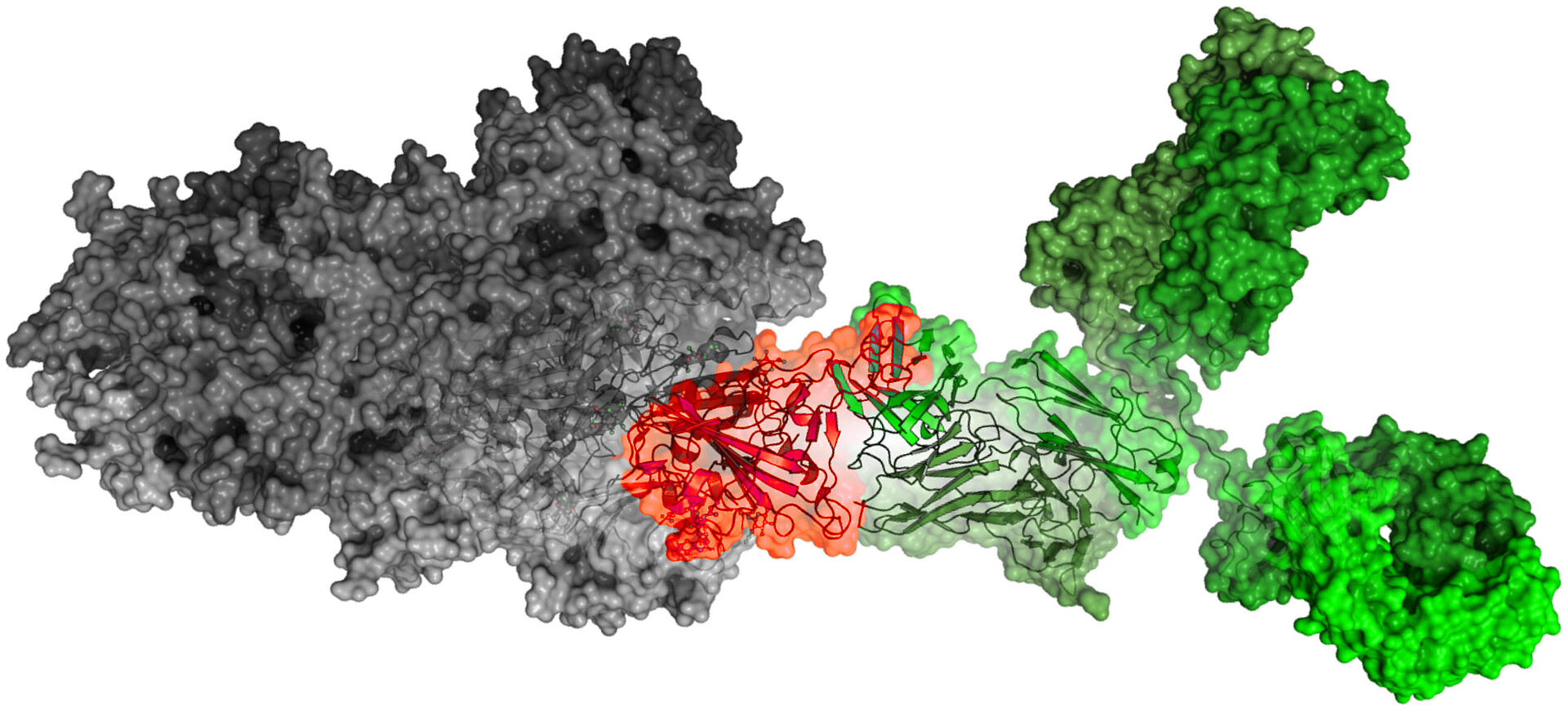
Braunschweig, Germany, June 22, 2021 - CORAT antibody COR-101 binds and inhibits all B.1.617 SARS-CoV-2 variants, including the fast proliferating “delta” variant
The newest laboratory tests indicate that COR-101 binds and inhibits the particularly aggressive new „delta” variant B.1.617.2, which is rapidly replacing the “alpha” variant B.1.1.7. On May 10th, the WHO classified the B.1.617.2 SARS-CoV-2 variant, first detected in India, as “of concern”. According to current data from the European Centre for Disease Prevention and Control (ECDC) the “delta” variant B.1.617.2 has replaced previous SARS-CoV-2 variants, including the “alpha” variant B.1.1.7, currently prevalent in Germany. According to ECDC analysis, the mutations likely increase transmissibility and decrease neutralization by convalescent plasma and currently approved therapeutic antibodies, explaining the sharp increase in the number of cases in India in recent weeks. The currently used vaccines obviously have different efficacies against the newly emerging variants. Also, while vaccines can protect healthy people, they cannot cure people already suffering from COVID-19; moreover, not everyone responds to vaccination. For these patient groups, CORAT Therapeutics GmbH is developing a drug that has an effect against SARS-CoV-2 directly.
Braunschweig, June 03, 2021 - CORAT secures 12,7 Mio € from Lower Saxony State, private investors, and the German Ministry of Research and Education (BMBF)
NBank Capital and private investors from Braunschweig, each having participated in previous financing rounds, renewed their investments. The current round of financing includes additional new investors from Braunschweig. Additionally, CORAT Therapeutics GmbH is to receive up to seven million euros from the federal funding program „Research and development of urgently needed therapeutics against SARS-CoV-2“ to support clinical development of COR-101. The goal of the current financing round is to secure funding for a Phase II clinical trial with the antibody drug candidate COR-101.
Lower Saxony State Secretary of Economics, Dr. Bernd Althusmann, comments:
"CORAT has completed all the necessary development steps on schedule so far. Now, it is important to maintain the fast development and to continue with the necessary clinical trials without delay. The government of Lower Saxony is excited to continue this success story."
4/23/2021 - First COVID-19 patients treated with COR-101 drug developed in Braunschweig, Germany.
Effective drugs against COVID-19 remain scarce in the current coronavirus pandemic. Even though the focus for months has been primarily on enabling rapid vaccination, there will be many people in the future who cannot be vaccinated, for example, due to concomitant diseases, or in whom the vaccination effect fails. Therefore, it is essential to quickly develop specific drugs for the treatment of COVID-19 disease.
The Corona Antibody Team (CORAT) of TU Braunschweig, led by Prof. Michael Hust and Prof. Stefan Dübel, and the biotechnology company YUMAB GmbH in Braunschweig have developed the virus-neutralizing antibody COR-101, which is highly effective against SARS-CoV-2 in laboratory tests. For the clinical development of COR-101, CORAT Therapeutics GmbH was founded with the support of the state of Lower Saxony, which has now started the first clinical trials in collaboration with t the University Hospital of Tübingen. Further clinical study centers are located in Braunschweig, Stuttgart (Robert Bosch Hospital), Leipzig and Dresden. The Phase Ib/II study is being conducted at these sites. In Phase-I, the tolerability of the antibody is initially being investigated in four dose cohorts. Phase II results on efficacy may form the basis for a marketing authorization application for the drug. Initial interim results are expected in the summer.
The antibody COR-101 differs in one important respect from the antibody preparations previously approved in the USA, which were procured by the German Federal Ministry of Health: COR-101 is specifically designed to treat hospitalized moderately to severely ill patients, for whom the U.S. antibodies must not be used because of their side effects. COR-101 avoids these side effects through a different, specially adapted molecular design.
Dr. Bernd Althusmann, Lower Saxony Minister of Economic Affairs, comments: "The promising antibody drug COR-101 shows that research made in Lower Saxony can provide an important building block on the way out of the pandemic. The clinical test phase that started yesterday gives hope that the Braunschweig project can soon save lives."
Further details:
press release of CORAT Therapeutics
Website of CORAT Therapeutics
more about the Mechanism of action of COR-101 here (animation) and here (crystal structure)

29.04.2021 - Video in DW television about COR-101 and CORA Therapeutics, featuring Dr. Andre Frenzel, CORAT Chief Scientific Officer
March 16, 2021 - CORAT Therapeutics GmbH obtained regulatory authorization for clinical phase I/Ib trial with the SARS-CoV-2 neutralizing human antibody COR-101.
The study (ClinicalTrials.gov ID: NCT04674566) is a randomized, double-blind, placebo-controlled, parallel-group, multicenter, first-in-human, phase I/Ib study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, immunogenicity, and efficacy of COR-101 in hospitalized patients with moderate to severe COVID-19.
"We are very happy to have reached this crucial step in COR-101 development. Many thanks to the support of the State of Lower Saxony and our private investors. We feel confident that COR-101 can fill the gap in the medical need for the treatment of hospitalized COVID-19 patients that have moderate and severe symptoms, where no other specific treatment is available at this time worldwide. The development of specific therapeutics to treat COVID-19 diseases is next to vaccination and testing an important pillar to effectively fight the pandemic., comments Dr. Andreas Herrmann, CEO of CORAT.
March 11, 2021 - Neutralizing anti-SARS-CoV-2 antibodies from immune cells of healthy donors
An international research team of the Technische Universität Braunschweig and the Helmholtz Centre for Infection Research (HZI), led by the Department of Biotechnology of the TU Braunschweig, reported the development of virus-neutralizing antibodies against SARS-CoV-2 in the journal Nature Communications. These antibodies prevent the viruses from entering host cells and were isolated from an antibody gene library that had already been produced from immune cells of healthy donors before the pandemic. The study shows how, in the future, active substances to previously unknown, emerging viruses can be produced very quickly without the need for patient-derived material.
original publication (open access)
18. Februar 2021 - A team of researchers from the Technische Universität Braunschweig, together with partners from the EU consortium ATAC (Antibody Therapy Against Corona), has published the results of studies on the longevity of the immune response of COVID-19 patients in the medical journal "Med" published by Cell Press.
more details (in german)
Original publication (PDF download)
December 18, 2020 - Human SARS-CoV-2 neutralizing antibody COR-101 demonstrates high efficacy in a COVID-19 animal model and is ready to be used in clinical studies to treat COVID-19 patients
CORAT Therapeutics GmbH today reports the successful conclusion of hamster disease model tests of their lead candidate COR-101 against COVID-19. "Our antibody induced recovery of SARS-CoV-2 infected hamsters within two days. In contrast, without treatment, the hamsters only started to reach the same health status after a full week." comments Dr. Andreas Herrmann, CEO of CORAT Therapeutics.
Treatment with COR-101 substantially reduced the weight loss after infection, which indicates the general health state. But since the main life-threatening effects of the virus are happening in the lung, the virus titer was measured directly in this organ which mainly contributes to lethality of SARS-CoV-2.
Dr. Herrmann says: "We are especially happy to report that COR-101 drastically reduced the amount of SARS-CoV-2 in the lung. Already after three days, an average of less than 1 % of the virus titer of the untreated control group could be detected after treatment with COR-101. This confirms the excellent neutralizing capability that we already measured in the laboratory. It demonstrates that COR-101 efficiently neutralizes the virus in a living organism and prevents disease progression".
In that respect, Dr. Herrmann also emphasized the difference in effector mechanisms compared to other antibodies: "In contrast to plasma therapy and the currently emergency approved antibodies in the US, our recombinant antibody is especially designed not to induce overshooting immune responses which contribute to the lethality in the lung. We achieved this by carefully removing the respective signals from the molecule before starting its production."
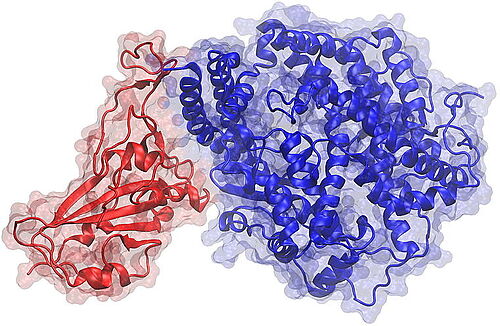
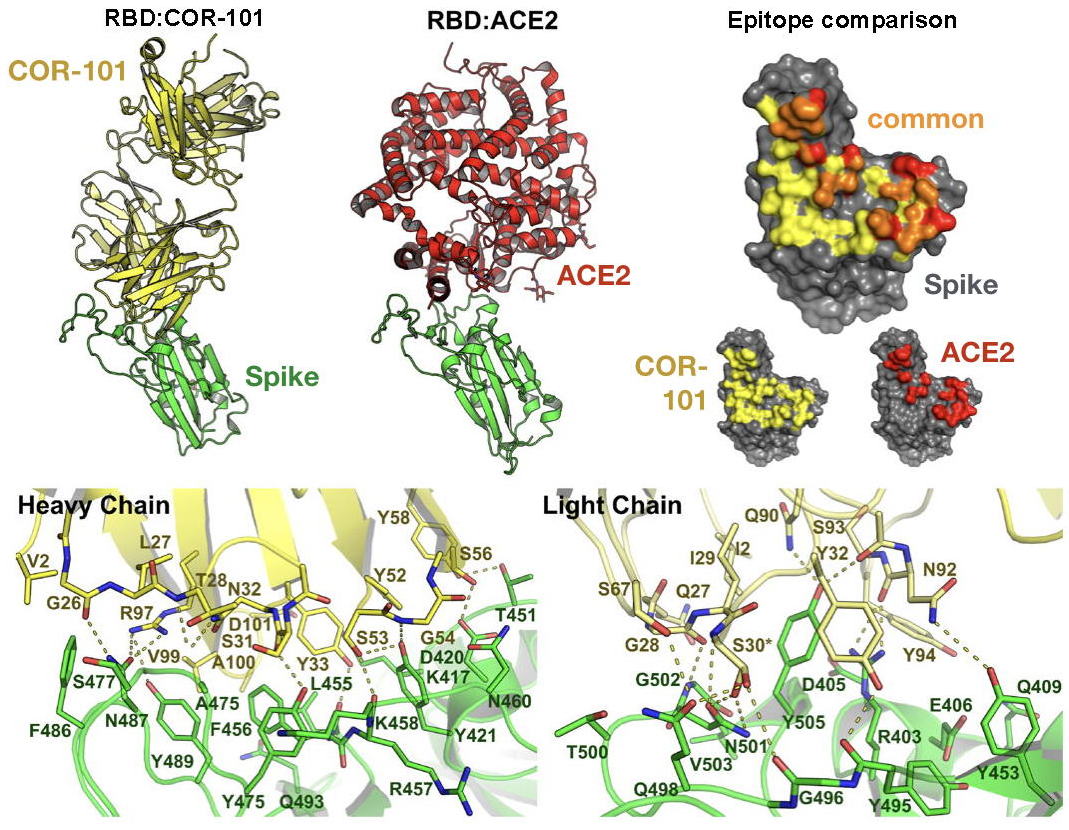
4.12.2020 - CORAT today published how COR-101 blocks SARS-CoV-2 and prevents infection with the coronavirus. The fully human antibody COR-101 showed an IC50 of 0.56 nM in a plaque-based live SARS-CoV-2 neutralization assay. The crystal structure of COR-101 in complex with SARS-CoV-2-RBD was solved at 2.0 A resolution showing that the antibody binds at the same region as the human coronavirus receptor ACE2 to SARS-CoV-2-RBD. In contrast to other published anti-SARS-CoV-2 antibodies, the binding of STE90-C11 is not blocked by known virus mutations, endowing COR-101 with higher intrinsic resistance to those possible escape mutants.
CORAT CEO Dr. Andreas Herrmann explains: "We know that a vaccine does not work in every person, and this is especially observed in older people. There are patients with other diseases that cannot be vaccinated as well. Unfortunately, these are precisely the two groups of people who usually have a higher risk of developing COVID-19. This is where COR-101 should help. Since COR-101 permanently occupies the essential contact point between the virus and our body, the virus can no longer use its "spike" proteins to infect us. We therefore expect COR-101 to be able to help all those who already have COVID-19 but whose immune system was unable to initiate its own antibody response."
Unlike the typical vaccines currently under investigation, antibody drugs do not require sophisticated freezing logistics, they are very stable and robust molecules. A century ago, doctors carried vials of antibodies in their pockets for weeks without refrigeration, so that they were always ready for use against tetanus or diphtheria, for example. COR-101 is therefore expected to have much simpler and cheaper logistics for the distribution than RNA vaccines.

1.12.2020 - In an event with Economics Minister Dr. Althusmann, Dr. Sabine Johannsen, State Secretary for Science and Culture and Stefan Muhle, State Secretary in Lower Saxony’s Ministry of Economics, Labour, Transport and Digitisation, CORAT Therapeutics won this year’s startup Award „Durchstarterpreis“ for corona solutions of the State of Lower Saxony.

18.11.2020 - CORAT lead product COR-101 ready for clinical trials
CORAT Therapeutics CEO Dr. Andreas Herrmann today reports the conclusion of the production campaign providing the first larger batch of COR-101 ready for the patients: „We are extremely happy and would like to thank everybody who helped to make this happen so quickly. With acute case numbers rising fast, CORAT hopes to make a difference by providing an option soon to directly treat the Coronavirus-Infected. We hope that the clinical studies now can commence very soon.“
28.9.2020 - CORAT wins the Innovation Award of the State of Lower Saxony 2020.

16.7.2020 - Fraunhofer ITEM and Corat Therapeutics GmbH start accelerated production of a passive vaccine against COVID-19.
16.6.2020 - With support from the German Federal State of Lower Saxony, CORAT Therpeutics was formed as a dedicated body to promote the further development of the antibody based COVID-10 medicine.
5.6.2020 - CORAT publishes the first substantial dataset describing fully human antibodies from heathly donors that are able to block the infection by SARS-CoV-2.
![[Translate to English:] CORAT antibodies blocking SARS-CoV-2 infection](/fileadmin/Redaktionsgruppen/Institute_Fakultaet_2/BBT-Biotech/Grafiken/CORAT/Spike3D.jpg)
4.5.2020 - CORAT member YUMAB, spinoff of the Department of Biotechnology of the TU Braunschweig, generated a neutralising coronavirus antibody protecting against SARS-CoV-2 infection, as confirmed by the Helmholtz Center for Infection Reserach
![[Translate to English:] CORAT member YUMAB generated protective coronavirus antibody](/fileadmin/Redaktionsgruppen/Institute_Fakultaet_2/BBT-Biotech/Grafiken/CORAT/Schirrmann-Frenzel-YUMAB.jpg)
CORAT is a consortium of academic and industrial partners with the mutual goal to develop a passive vaccination immunotherapy against COVID-19 employing novel fully human monoclonal antibodies neutralizing SARS-CoV2.
Coordination: Prof. Dr. Stefan Dübel, Prof. Dr. Michael Hust (TU Braunschweig).
Coordination CORAT Therapeutics GmbH: Dr. Andreas Herrmann
A large set of more than 750 human monoclonal antibodies recognizing SARS-CoV2 Spike (S) protein isolated by antibody phage display from human antibody libraries from healthy donors and convalescent COVID-19 patients underwent a fast track development process to identify lead molecule with optimal drug features for a passive vaccine. Thanks to innovative approaches and the most modern methods, we were able to provide the drug candidate (COR-101) in a much shorter time than was previously possible, with all the necessary safety and quality requirements for drugs controlled by the PEI (Paul Ehrlich Institute, responsible for clinical testing). We have thus generated a drug based on an endogenous human substance that is being tested in clinical trials as an immunotherapy (passive vaccine) for the treatment of acute COVID-19. In contrast to other antibodies against SARS-CoV-2, COR-101 may be used in particular for hospitalized patients. However, if successful, this drug could also be used prophylactically to protect medical caregivers, pre-diseased patients or at-risk populations.
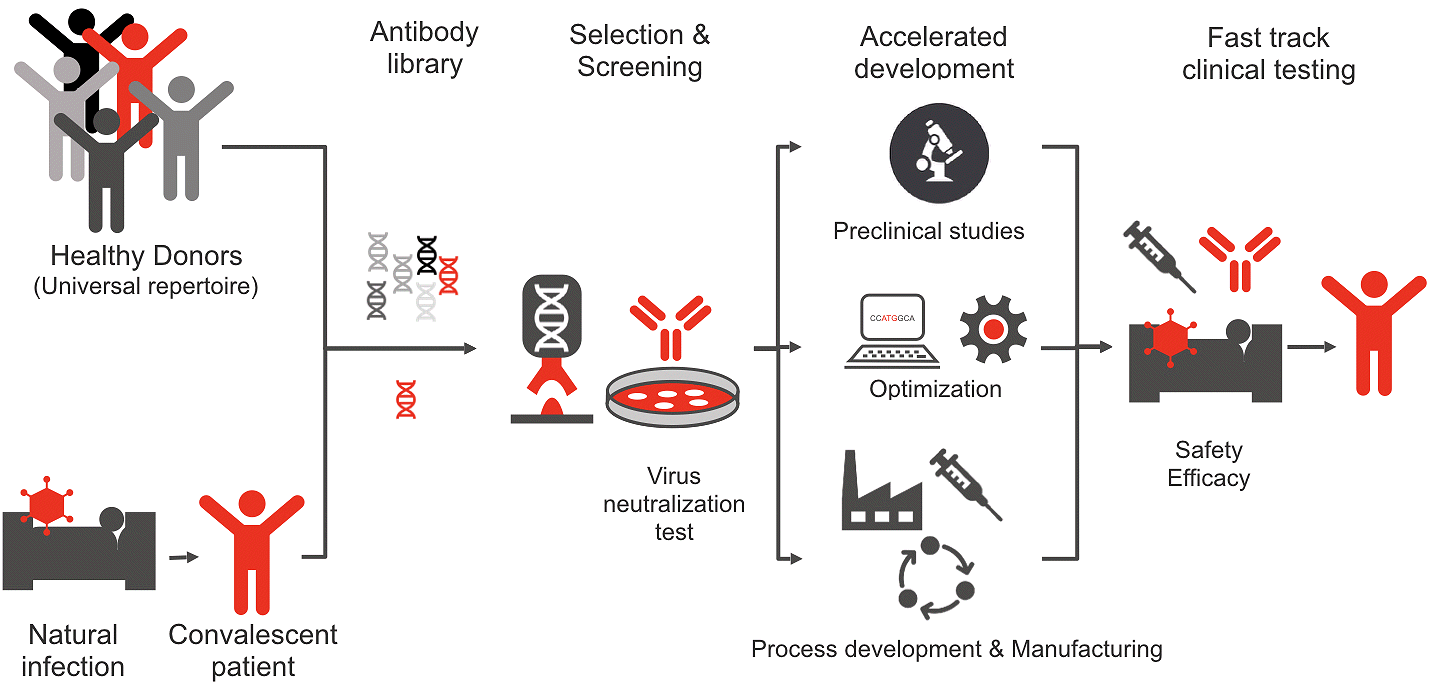
Recombinant human virus-neutralizing antibodies are the active component of the immunotherapy (also passive vaccine). Antibodies (immunogobulins, IgG) are molecules made by our own body to protect us against infections.
The CORAT antibody neutralize SARS-CoV2 after injection and immediately provides the protection in exactly the same way like antibodies would do from our own body once they are generated by the infection or by vaccination. The CORAT antibody stops the virus and protects the patients until their immunity generates their own antibodies. This drug should be very well tolerated, because it's mode of action and general structure represents the human antibodies (“fully human”) we develop by ourselves after an infection - actually, its encoding sequences were taken out of a convalescent COVID-19 patient.
Due to the long serum half time, the CORAT antibody could also be given in an early and acute stage of SARS-CoV2 infection with immediate antiviral effect and long protection until the body has the time to produce enough of its own IgG.
Other antibody drugs have already demonstrated the principle of virus neutralisation and are emergency approved in the USA. However, our COR-101 has some advantages over these antibodies due to its modified Fc region, which does not further stimulate the exaggerated immune response that is problematic in COVID-19 patients. Consequently, it is approved to be tested for the treatment of hospitalized COVID-19 patients with moderate to severe disease where the other products are not approved to be used.
Federal Research Secretary Anja Karliczek: "In addition to the availability of vaccines, the successful development of effective therapeutics is crucial to managing the corona pandemic in the long term. Even with a high vaccination rate, we must unfortunately expect that people will continue to contract COVID-19. " (Source: PM 14.06.2021 BMBF).
A typical vaccine ("active vaccine”) consisting of dead or partial virus material cannot heal COVID19 patients. The reason is that active vaccines induce an immune response - typically the generation of antibodies (IgG) - like the infection does itself. However, the development of protecting antibodies takes up to 2-3 weeks, too long for patients with a severe infection to survive. Therefore, patients that are already infected do not really benefit from such an active vaccine. The same is true for medical care personnel in emergency situations, because immunological protection starts only several weeks after the vaccination.
In contrast, CORAT antibody immunotherapy (also named "passive vaccine") supplements the not yet existing antibodies in the patient's body from the very time of injection, thus helping to lower the virus burden immediately. An active vaccine can protect healthy persons after some weeks, but it does not help patients already infected. Further, it cannot provide immediate protection to risk groups and exposed medical care personnel. CORAT antibodies can fill this treatment gap and thus perfectly complement virus-based vaccines.
Acute severe cases will be seen in our clinics for a long time to come, and a medication to help these patients is urgently needed.
Serum therapy is established since 120 years and well tolerated
Recombinant neutralising antibodies are a proven class of drugs
There is already an approved antibody neutralizing drug against lung viruses, which eliminates the disadvantages of serum therapy, and shows how well antibodies can be tolerated.
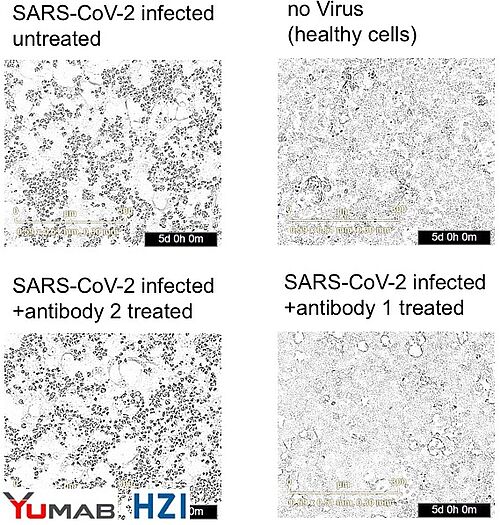
Infection of cells (in cell culture) with SARS-CoV2 isolates from a COVID19 patient lead to their lysis, indicated by rounding and loss of confluence (upper left panel). Uninfected cells (upper right) grow normally (no dead round dots). Lower right: Adding the CORAT antibody from Yumab to infected cells completely protected the cells from infection for 5 days, while a negative control antibody (lower left panel) did not protect the cells (Fotos: light microscopy). (Data: HZI, Prof. Čičin-Šain).
Numerous other human antibodies with similar activity were discovered, and compared for optimal drug properties. COR-101 was identified as the best compound at the end of these extensive set of experiments.
Originally initiated by Prof. Stefan Dübel, YUMAB founder and head of the Biotechnology Department of the University of Braunschweig and his colleague Prof. Michael Hust, together with Prof. Gundram Jung of University of Tübingen, CORAT rapidly expanded to a consortium of more than 30 scientists and clinicians from academic institutions and biotech and pharma industry. CORAT members represent competence from infection research, antibody development, preclinical testing and manufacturing to bedside and have joined forces to supply acute COVID19 patients with a well-tolerated treatment to eliminate SARS-CoV2.
Prof. Michael Hust (TU Braunschweig) is part of the EU Horizon2020 COVID19 consortium "ATAC". We are also grateful for rapid emergency funding from the Land Niedersachsen, and funding by the Deutsche Herzstiftung e.V., Frankfurt a.M.
Since 16.6.2020, the preclinical and clinical development is pursued by CORAT Therapeutics GmbH, a company founded for that purpose as a spin off of YUMAB with support by the State of Lower Saxony. Please refer to their website for most current informations.
...finally, please feel free to visit Abcalis, another award winning spin off of TU Braunschweig, providing animal free antibodies against the SARS-CoV2 spike and N proteins (already 100% compliant to the new recommendations of EU (EURL ECVAM JRC120199) / EU Directive 2010/63/EU).
Abcalis' anti-SARS-CoV-2 antibodies are currently in development for rapid antigen detection tests with partner companies.
Prof. Dr. Stefan Dübel / Prof. Dr. Michael Hust
Technische Universität Braunschweig, Institute of Biochemistry, Biotechnology and Bioinformatics
Spielmannstr. 7, 38106 Braunschweig, Germany
Phone: +49-531-391-5731, Fax: +49-531-391-5763
biotech@tu-bs.de
CORAT Therapeutics GmbH: Dr. Andreas Herrmann
For the most recent list of Publications, please also check here. Selected Papers:
Development of human antibody COR-101 (clinical development: CORAT Therapeutics)
Fully human SARS-CoV-2 neutralising antibodies from healthy donors:
Immune response to SARS-CoV-2 Infections in patients
Rapid SARS-CoV-2 antigen production in insect cells