Once migrated and differentiated, neurons have to remain stable and fulfill their function life-long. Thus proper homeostasis of neurons is essential to maintain a functional brain and thus control over body functions.
Neurodegenerative diseases such as Alzheimer’s Disease or Morbus Parkinson result in the progressive loss of specific neuronal populations leading to severe neurological deficits.
As no causative cures are currently available such neurodegenerative diseases are eventually lethal.
While the origin of many neurodegenerative diseases has a strong environmental component, also genetic factors are known predisposing or causing neurodegeneration.
These genetic variations can be used to genetically model human diseases in animals for analyzing disease etiology or for developing therapeutic approaches.
In zebrafish such genetic disease models allow for monitoring disease onset and progression on the cellular level in a non-invasive manner, which we use to understand the underlying cell biological mechanisms of neurodegeneration.
Furthermore, with the large number of small and transparent progeny in an aqueous environment, zebrafish are ideally suited for pharmaceutical and toxicological studies.
We therefore use zebrafish embryos from genetic models of neurodegenerative diseases for screening, evaluating and validating pharmaceutical compounds.
Both their effectiveness in interfering with disease initiation and progression as well as their potential side effects can be tested directly in vivo.
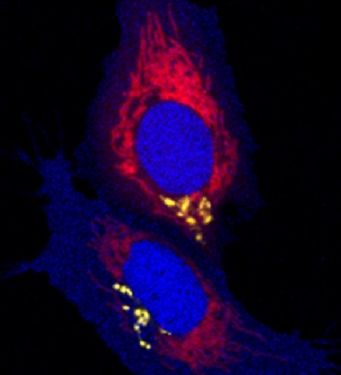
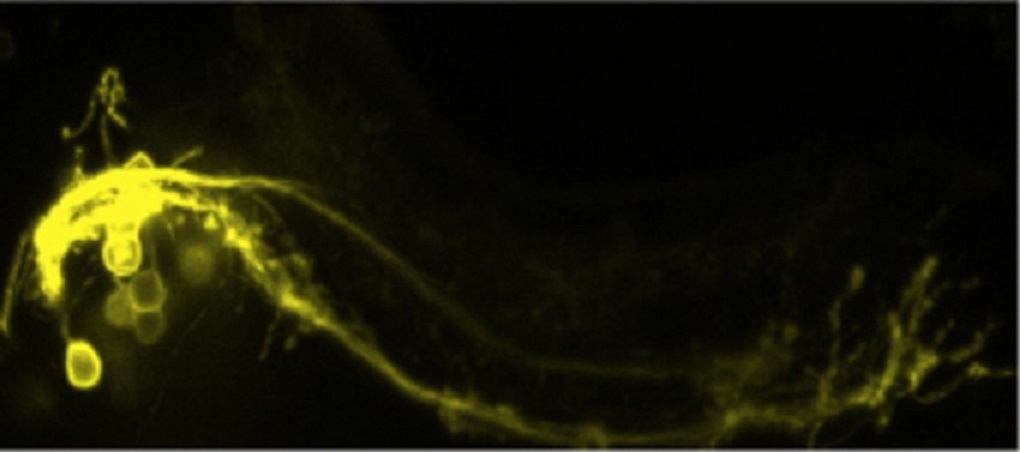
Genetic inheritance is the major cause of neurodegenerative diseases of the cerebellum.
Usually, such fatal diseases have a mid-life onset and result in progressive locomotive disabilities. These behavioral phenotypes are easy to examine in zebrafish as the cerebellar functions are conserved among vertebrates.
Currently, we are establishing genetic models of neurodegenerative diseases of the cerebellum for cell biological, physiological, behavioral and pharmacological characterization.
These models are accessible for bio-imaging analysis and thus will permit in vivo monitoring of disease initiation and progression as well as conception and validation of therapeutic approaches at cellular resolution.
Selected publications:
Namikawa, K., Dorigo, A., & Köster, RW. (2019). Neurological Disease Modelling for Spinocerebellar Ataxia Using Zebrafish. (Article Commentary) J Exp Neurosci. 13:1-5.
Namikawa, K., Dorigo, A., Zagrebelsky, M., Russo, G., Kirmann, T., Fahr, W., Dübel, S., Korte, M., & Köster, RW. (2019) Modeling Neurodegenerative Spinocerebellar Ataxia Type 13 in Zebrafish Using a Purkinje Neuron Specific Tunable Coexpression System. J Neurosci.39(20):3948-3969.
Flinn, L., Keatinge, M., Bretaud, S., Mortiboys, H., Matsui, H., De Felice, E., Woodroof, H. I., Brown, L., McTighe, A., Söllner, R., Allen, C. E., Heath, P. R., Milo, M., Muqit, M. M. K., Reichardt, A. S., Köster, R. W., Ingham, P. W., Bandmann, O. (2013). TigarB causes mitochondrial dysfunction and neuronal loss in PINK1 deficiency. Annals of Neurology 74: 837-847.
Walker, S. L., Ariga, J., Mathias, J. R., Xie, X., Coothankandaswamy, V., Bhalla, K. N., Distel, M., Köster, R. W., Parsons, M. J., Saxena, M. T., Mumm, J. (2012). Automated Reporter Quantification in vivo (ARQiv): High-throughput screening method for reporter-based assays in zebrafish. PLoS One 7: e29916. doi:10.1371/journal.pone.0029916.
Flinn, L., Mortiboys, H., Volkmann, K., Köster, R. W., Bandmann, O. (2009). Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio). Brain 132: 1613-1623.
Paquet, D., Bhat, R., Mandelkow, E.-M., Berg, S., Hellberg, S., Lindquist, J., Distel, M., Köster, R. W., Schmid, B., Haass, C. (2009). A novel Tau transgenic zebrafish model for drug discovery. Journal of Clinical Investigations 119: 1382-1395.