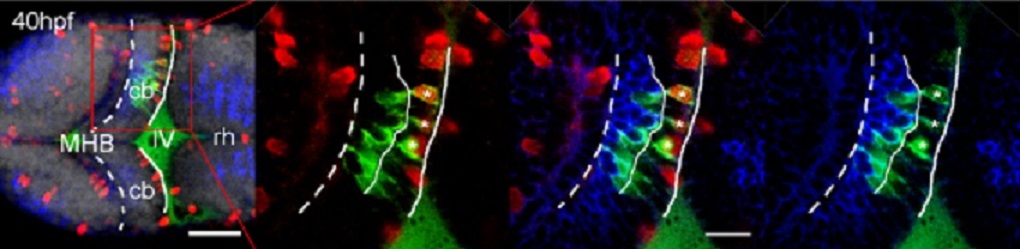
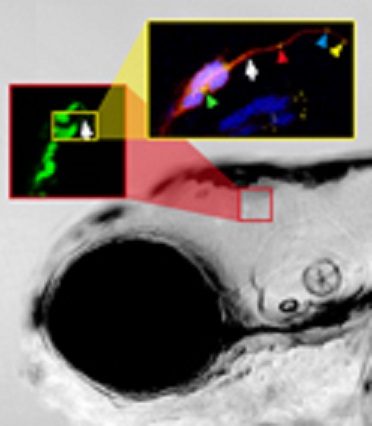
The migration of neurons requires a carefully choreographed combination of the onset of migration in proper numbers of cells and direction, successful pathfinding during migration followed by the final positioning of migrating neuronal precursors at their target tissue.
Faced with the complexity of studying these interleaved processes of neuronal development, a powerful approach is to combine recent advances in optical physics of high resolution imaging with innovative tools of molecular genetics to follow neuronal cells within the living organism.
Due to their transparency, fast embryogenesis and genetic accessibility zebrafish embryos are used as model organism focusing on the cerebellum inside the brain.
We have established in vivo culture methods to incubate zebrafish embryos on the microscope stage allowing neuronal precursors of the cerebellum to be continuously followed by in vivo confocal microscopy for up to three days.
This three-dimensional imaging over time elucidated the detailed migratory pathway of cerebellar neuronal precursors, their proliferation pattern, the individual behavior of cells during different phases of their migratory route and the interactions of cells while migrating.
In addition, multi-color imaging revealed the subcellular orchestration of migration and showed how individual cells coordinate the movements of their organelles to ensure directional Migration.
Taking advantage of the genetic accessibility of zebrafish both wild-type and transgenic neuronal precursors in wild-type as well as mutant embryos are now being followed through in vivo imaging as they migrate and adopt phenotypes thereby addressing the molecular mechanisms that govern these crucial events of vertebrate brain development.
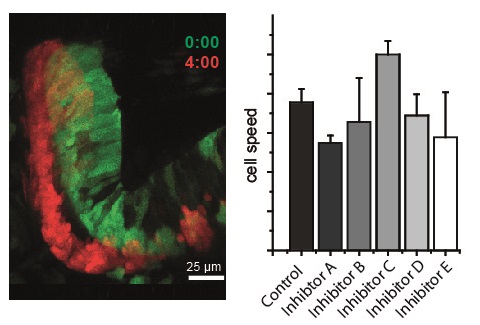
Current topics in the lab are:
1. How does activity influence migrating neurons?
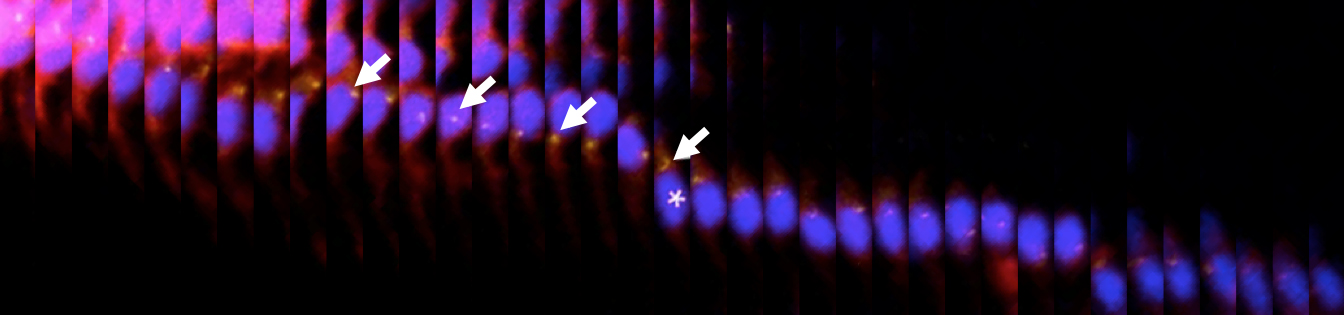
Neurons are governed in their migration by different types of activity. Using modern imaging techniques, such as Calcium imaging, optogenetics, and the adaptation of a track analysis software to trace cells in tissues that shift their position in the developing embryo, we have recently identified the neurotransmitters which control the migration of cerebellar neurons.
We also find that their action appears spatially confined, and have summarized these findings in a map of the cerebellum indicating regions of influence.
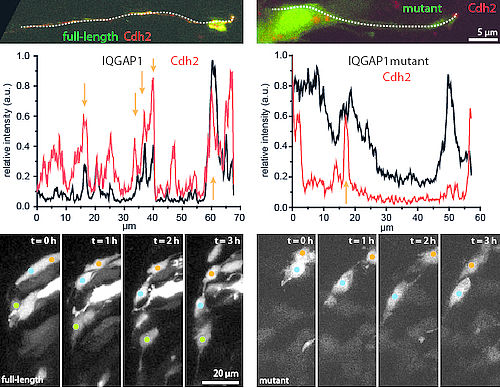
2. How is the signal for migration transmitted to the cytoskeleton?
We have already identified a regulatory factor of cell migration, the cell-cell adhesion protein Cadherin-2. Ultimately, Cadherin-2 needs to act on the cytoskeleton to produce forward motion.
We are currently testing proteins of the IQGAP family which could act as bridge between the regulatory factor and the cytoskeleton.
This study involves the in vivo observation of co-localization between Cadherin-2 and the IQGAPs and their mutants, as well as testing the effect of the mutants in migrating cerebellar cells.
3. What is the role of microtubules in neuronal migration?
Several models exist how the cytoskeleton produces the necessary forces for forward movement. The cytoskeleton consists of several elements, of which actin and microtubules are the best studied. Cell migration in general is a property mostly of the actin cytoskeleton, yet previous studies have attributed an essential role for microtubules in this process specifically in neurons in the past. Early models propose that microtubules contribute forces directly to moving cells forward, while more recent studies suggest that the contribution of microtubules to neuronal migration lies mostly in their regulatory effects. As microtubules are well-known to distribute cargo throughout a cell, Cadherin-2 presumably among it, we are interested in probing the involvement of microtubules in cerebellar neuron migration in vivo.

The current research is generously funded by Marie Curie Actions of the European Commission:
Selected publications:
Volkmann, K., Rieger, S., Babaryka, A., Köster, R. W. (2008). The zebrafish cerebellar rhombic lip is spatially subdivided in producing granule cell populations of different functional compartments. Developmental Biology 313: 167-180.
Rieger, S., Senghaas, N., Walch, A., Köster, R. W. (2009). Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biology 7: e1000240. doi:10.1371/journal.pbio.1000240.
Distel, M., Hocking, J., Volkmann, K., Köster, R. W. (2010). The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. Journal of Cell Biology 191: 875-890. (Summarizing Biosights Video)
Theisen, U., Hennig, C., Ring, T., Schnabel, R., Köster, R. W. (2018). Neurotransmitter-mediated activity spatially controls neuronal migration in the zebrafish cerebellum. PLoS Biology 16: e2002226.