The bifidogenic properties of fructooligosaccharides (FOS) produced from sucrose and galacto-(GOS) or xylose-containing oligosaccharides have been studied in detail[1, 2, 3 and 4]. The focus of this project lies on the immobilization of biocatalysts which participate in the synthesis of prebiotic oligosaccharides. We want to identify suitable biopolymers for achieving an efficient immobilization via electrospinning while maintaining the enzymatic activity over a long period of time. Furthermore the kinetic properties of the native and immobilized enzymes will be studied. To optimize the production of prebiotic oligosaccharides we want to design a bienzymatic or trienzymatic system which allows the continuous production of oligosaccharides in larger scales.
Electrospinning provides a versatile and reasonable method for generating continuous nanofibers from a variety of natural or synthetic polymers. It utilizes electric forces to produce fine fibers from solutions or melts with diameters ranging from a few micrometers to several nanometers. Electrospinning offers also numerous possibilities to form novel nanostructures with controllable pore sizes, diversified compositions or remarkable length.
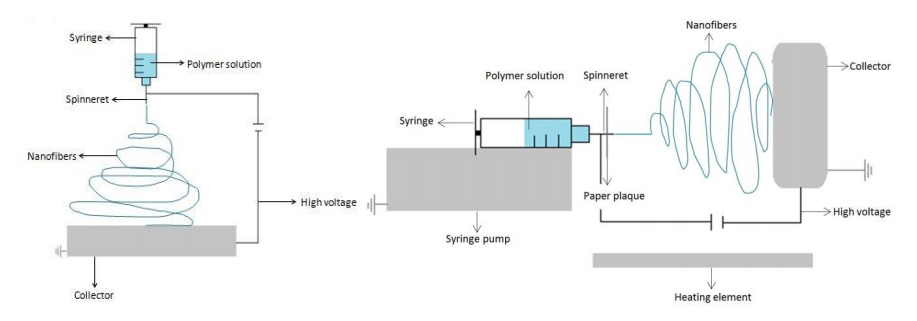
Nanofibers with more complex architectures, such as core-shell-fibers or fibers with hollow interiors can be achieved by using special electrospinning methods. It is also possible to entrap anorganic material, (bio-) catalysts or cells for application in many pharmaceutical and food technology processes.
![SEM-images of electrospun nanofibers from (a) polystyrene (PS)//DMF/MEK(2:1) solution (b) polycaprolactone (PCL) // dichlormethane (DCM) (c) Co-Electrospinning of PCL/polyethylene glycol (PEO).[5]](/fileadmin/Redaktionsgruppen/Institute_Fakultaet_2/ITC/ITC_HJ_Media/ITC_HJ_AnnCatherine_Grafik2.png)
Literature
[1] Hsu, Cheng-Kuang; Liao, Jiunn-Wang; Chung, Yun-Chin; Hsieh, Chia-Pei; Chan, Yin-Ching (2004): Xylooligosaccharides and Fructooligosaccharides Affect the Intestinal Microbiota and Precancerous Colonic Lesion Development in Rats; J. Nutr. 134 (6), pp 1523–1528.
[2] Ito, M.; Deguchi, Y.; Miyamori, A.; Matsumoto, K.; Kikuchi, H.; Kobayashi, Y. et al. (1990): Effects of Administration of Galactooligosaccharides on the Human Faecal Microflora, Stool Weight and Abdominal Sensation; Microbial Ecology in Health and Disease 3 (6).
[3] HIDAKA, Hidemasa; TASHIRO, Yasuhito; EIDA, Toshiaki (1991): Proliferation of Bifidobacteria by Oligosaccharides and Their Useful Effect on Human Health; Bifidobacteria Microflora 10 (1), pp 65–79.
[4] Fanaro, Silvia; Boehm, Günther; Garssen, Johan; Knol, Jan; Mosca, Fabio; Stahl, Bernd; Vigi, Vittorio (2005): Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: A review: Acta Pædiatrica 94 (449), pp 22–26.
[5] Gabrielczyk, J. (2015): Institute of Technical Chemistry; TU Braunschweig.
