

In den Studiengang "Pharmaingenieurwesen" fand zum WiSe 2021/22 letztmalig eine Immatrikulation statt.
Zum Wintersemester 2022/23 startet der neue Masterstudiengang "Pharmaverfahrenstechnik". Nähere Informationen dazu finden Sie in Kürze an dieser Stelle.
Dokumente und Downloads zum Studiengang (Zulassungsordnung, Prüfungsordnung, Modulhandbuch u.a.) finden Sie auf der Seite Dokumente Pharmaingenieurwesen.
Der Masterstudiengang Pharmaingenieurwesen verbindet verfahrenstechnische und pharmazeutische Inhalte. Diese Kombination von Kenntnissen wird insbesondere auf dem Weg vom Wirkstoff zur fertigen Arzneiform benötigt, sowohl hinsichtlich einer wirksamen, auf den Produktionsprozess abgestimmten Formulierung, als auch der Übertragung eines Laborprozesses in den Produktionsmaßstab.
Charakteristische Aufgaben von Pharmaingenieur*innen sind daher die Entwicklung und Optimierung von Produktions- und Formulierungsverfahren, die Planung von Produktionsanlagen, die Qualitätssicherung sowie die Qualifizierung und Validierung von Anlagen und Prozessen. Mit klassischen Studiengängen sind diese Aufgaben erst nach langen Jahren Berufserfahrung möglich, da klassischer Verfahrenstechniker*innen zunächst die einschlägigen Vorschriften und ein Pharmazeut*innen das verfahrenstechnische Know-How erlernen müssen.
Durch dieses Studium werden die Absolvent*innen deutlich früher in die Lage versetzt Aufgaben im Grenzgebiet zwischen Pharmazie und Verfahrenstechnik zu übernehmen und auf diese Weise die Bereiche der Forschung und der Produktion in einem pharmazeutischen Betrieb oder im universitären Umfeld zu verbinden.
Der Studiengang richtet sich an Absolvent*innen von Studiengängen mit verfahrenstechnischen und/oder pharmazeutischen Inhalten. Um diesem sehr heterogenen Ausbildungstand der Studierenden gerecht zu werden, erfolgt, primär im ersten Semester, der Bereich Fachkomplementäre Qualifikationen. In diesem erlernen die Studierenden die fehlenden Grundlagen, bevor im zweiten und dritten Semester einige Pflichtvorlesungen die Kernkompetenzen für Pharmaingenieur*innen vermitteln.
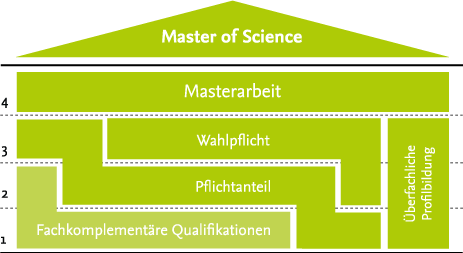
Begleitet wird der Pflichtbereich von fachübergreifenden Qualifikationen sowie einem Wahlpflichtbereich, in dem die Studierenden ihre persönlichen Interessen vertiefen können. Der Abschluss des Studiums erfolgt über eine Masterarbeit, in der konkrete Fragestellungen aktueller Forschungsprojekte selbstständig bearbeitet werden.
*** Bitte beachten Sie den o.g. aktuellen Hinweis für Studieninteressierte zum WiSe 2022/23 ***
Der Masterstudiengang Pharmaingenieurwesen ist ein konsekutiver Studiengang für Studierende, die einen ersten berufsqualifizierenden Studienabschluss (z.B. Bachelor) in einem naturwissenschaftlich-pharmazeutisch und/oder einem ingenieurwissenschaftlich-verfahrenstechnisch orientierten Studiengang erhalten haben. Ein Studiengang wird als Studiengang mit pharmazeutischen und/oder verfahrenstechnischen Inhalten eingeordnet, wenn mindestens 50 Leistungspunkte in pharmazeutischen und/oder verfahrenstechnischen Fächern erbracht wurden.
Durch das Angebot des individuell zu gestaltenden Grundlagenteils steht der Studiengang auch Studierenden offen, deren Vorbildung auf einen der Bereiche, Pharmazie oder Verfahrenstechnik, beschränkt ist.
Zusätzlich zu den üblichen Bewerbungsunterlagen ist ein Motivationsschreiben einzureichen.Das Motivationsschreiben wird benotet und mit der Note des Studiengangs verrechnet.
Technische Universität Braunschweig
Fakultät für Maschinenbau
-SERVICEteam-
Schleinitzstr. 20
38106 Braunschweig
Tel: +49 (0)531 391 4040
service-fmb(at)tu-braunschweig.de
Informationen zu den Lehrveranstaltungen und Klausurterminen der pharmazeutischen Institute (Fak. für Lebenswissenschaften) für Studierende im Master Pharmaingenieurwesen