Fungal ß-glucans: Schizophyllan and Scleroglucan
Prof. Dr. Udo Rau (ret.)
The filamentously growing fungi Schizophyllum commune and Sclerotium rolfsii secrete extracellular polysaccharides, called Schizophyllan and Scleroglucan, respectively, during growth. Both gums possess an identical primary molecular structure consisting of a backbone chain of 1,3-ß-D-glucopyranose units linked with single 1,6-bounded ß-D-glucopyranoses at about every third glucose molecule in the basic chain. The molecular weight varies between 6 and 12. A wide range of carbon sources sustain both growth and ß-glucan formation. Simple mono- or disaccharides, such as glucose, xylose, mannose, sucrose, maltose but also galactose are utilized as well as polysaccharides as starch or xylan. However, best results were achieved with glucose as carbon and energy source. Shear stress as a result of the impeller type used and rotation speed applied as well as an optimum but not maximum oxygen supply are the key factors for enhanced production. Batch cultivations performed under oxygen limitation resulted maximum yields of 13 g/L for both ß-glucans. Both fungi underlie an glucose-induced repression of the formation of ß-glucanases. Therefore, cultivations were terminated after glucose consumption. A major difference between both fungi exists in the formation of diverse by-products. While S. commune forms ethanol S. rolfsii does not possess a fermentation pathway, however, secretes oxalic acid by the glyoxylate pathway. In oxygen limited chemostats with biomass feedback maximum productivities of 95 g/Ld were attained. Optimized process and filtration conditions resulted a cellfree and undiluted ß-glucan solution at the outlet of the bioreactor. The subsequent downstream process was also carried out by cross flow filtration techniques. Aqueous solutions of the ß-glucans show thixotropic, pseudoplastic and viscoelastic behaviour. Ciba Specialty Chemicals (Grenzach/Basel) distributes Scleroglucan under the trade mark Tinocare™GL which can be used for following applications:
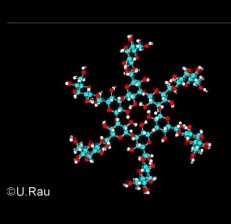
Fig. 3
Top view ot the triple helical arrangement of Scleroglucan.
The ß-1,6-linked glucose units extend perpendicular from every third backbone sugar.
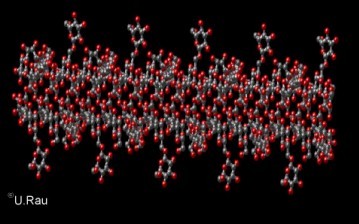
Fig. 4
Side view ot the triple helical arrangement of Scleroglucan
Production of biosurfactants using renewable resources and related applications
Prof. Dr. Siegmund Lang and PD Dr. Udo Rau
Biosurfactants have gained notably attraction during past years due to their low toxicity, biodegradebility and structural diversity and are formed by several microorganisms and/or enzymatic treatment. They cover a broad range of potential industrial applictations and are found in enhanced oil recovery, crude oil drilling, lubricants, surfactant-aided bioremediation, health care and food. Moreover, they show are used as pharmaceutics. Among the biosurfactants the glycolipids cover a broad area of these amphiphile structures. Glycolipids are low molecular weight products (< 1,500 D) consisting of a carbohydrate (mono-, di- or oligosaccharide) and one or more lipophilic moieties containing saturated, unsaturated and/or hydroxylated fatty acids or fatty alcohols. Some fungi and bacteria (e.g. Candida bombicola, Ustilago maydis, Tsukamurella spec. ) are able to grow on carbohydrates and/or triglycerides (vegetable oils and fats) and fatty acids, respectively, as well as to use these carbon sources simultaneously for the production of glycolipids. A scale-up of these bioprocesses was conducted up to a working volume of 300 L. Fig. 1 shows as example structures of a sophoro lipid and a mannosylerythritol lipid.
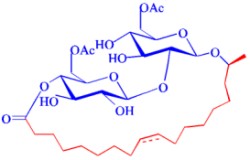
Fig. 1
Acetylated and ß-1'-4''-lactonized sophorolipid containing a 17-OH-oleic acid.
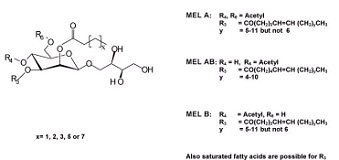
Fig. 2
2,3-Di-O-alkanoyl-ß-D- mannopyranosyl-(1-4)-O- meso-erythritol (mannosylerythritol lipid).
Due to their amphiphilic structure these glycolipids reduce the surface and interfacial tension of water/air and water/n-hexane, respectively. They are attractive compounds concernig their biological origin compared to common chemically synthesized surfactants. Biosurfactants are used in:
Using marine biotechnology to develop new active ingredients
Prof. Dr. Siegmund Lang (ret.)

In contrast to certain macroorganismic marine organisms, marine microorganisms have came into the spotlight as a new source of natural substances. Our research group cultivated bacteria from the North Sea and the Mediterranean Sea, which were previously associated with sponges (see picture above) or algae. After strain identification, low-molecular natural substances were produced in a 50 L bioreactor, and after purification tested in bioassays for antibiotic, anti-tumor, anti-viral or neuroprotective effects. In the case of one of these bacteria, we were able to isolate an active ingredient that is antimicrobial in its native form and additionally provides anti-tumor effects after slight chemical modification.