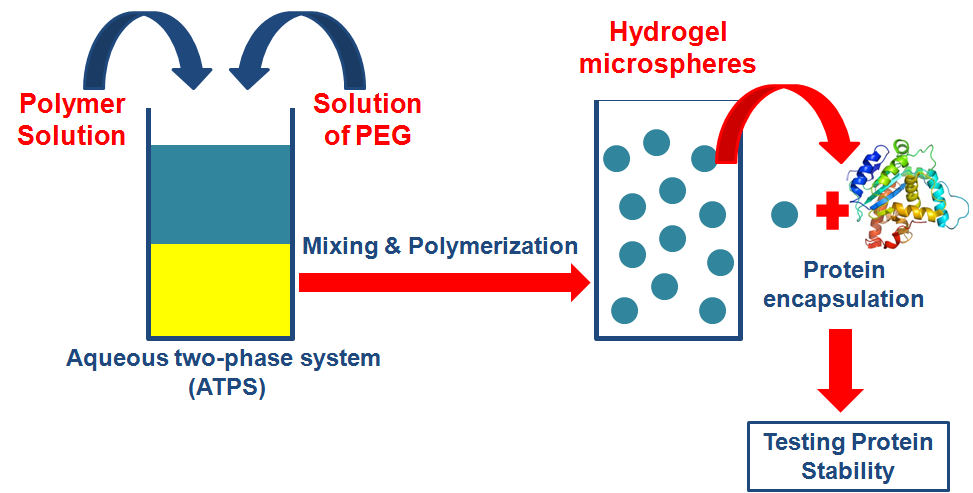
Arthritis constitutes one of the major serious conditions that negatively affect the patients’ quality of life. Arthritis is associated with comorbid conditions, physical and mental debilitation and negative influence on life expectancy. Thus, great care is directed to effectively treat this medical condition. Unfortunately, conventional medications such as oral analgesics do not always provide an effective cure. Thus, novel approaches have been adopted that involve the use of monoclonal antibodies that inhibit the inflammatory mediators, consequently delay disease progress. However, the delivery of proteins into the human body faces many barriers such as enzymatic degradation in addition to undesirable adverse-effects. Thus, it was mandatory to use a carrier that effectively encapsulate the protein and offer adequate protein protection. Hydrogels (HGs) were selected for this purpose due to their biocompatibility and the feasibility to modify their structures to control the protein release. HG micro-particles are of distinct interest for this aim. Such microparticles are accessible via water-in-water emulsion systems. However, proteins may encounter instability when encapsulated in such polymer-based carriers or during the formulation of micro-particles. Thus, the purpose of the present study is to prepare HG-based systems using various polymers that can efficiently encapsulate the protein while maintaining its stability.