Research interest : Neurological Disease Modeling in Zebrafish
The evidence that approximately 80 % of human disease related genes are conserved in the zebrafish genome provides us with the chance to generate genetically inherited disease models not only for understanding the disease etiology, but also for evaluating potent compounds against human diseases.
Since zebrafish larvae are maintained in an aqueous environment in multi-well plates where chemicals can be easily applied, it is possible to study and validate drug effectiveness in terms of therapeutic concentrations, side effects and feasibility to cross the blood brain barrier.
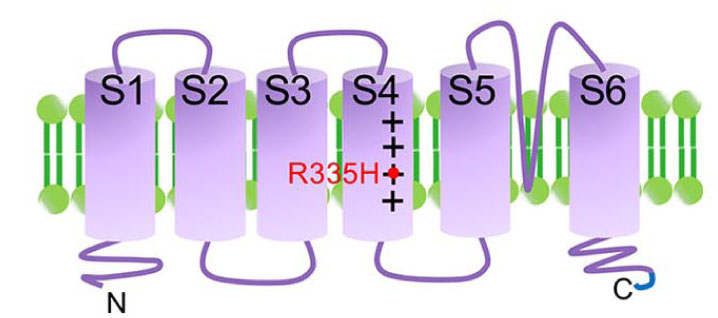
By making use of the established disease zebrafish, we are currently investigating: (1) cell biology of disease affected neurons, and (2) neuron-glia communication during progression of neurodegeneration in vivo.
Furthermore, our model will enable us to (3) validate the effectiveness of potent compounds towards mitigating these untreatable neurodegenerative diseases.
Please feel free to contact me if you get interested in our disease zebrafish models, or tools we developed.

To take these advantages of zebrafish, we have been working on a project aiming to develop zebrafish disease models for Spinocerebellar Ataxia (SCA), a class of neurodegenerative diseases, in which patients display progressive cerebellar atrophy and suffer from loss of motor coordination.
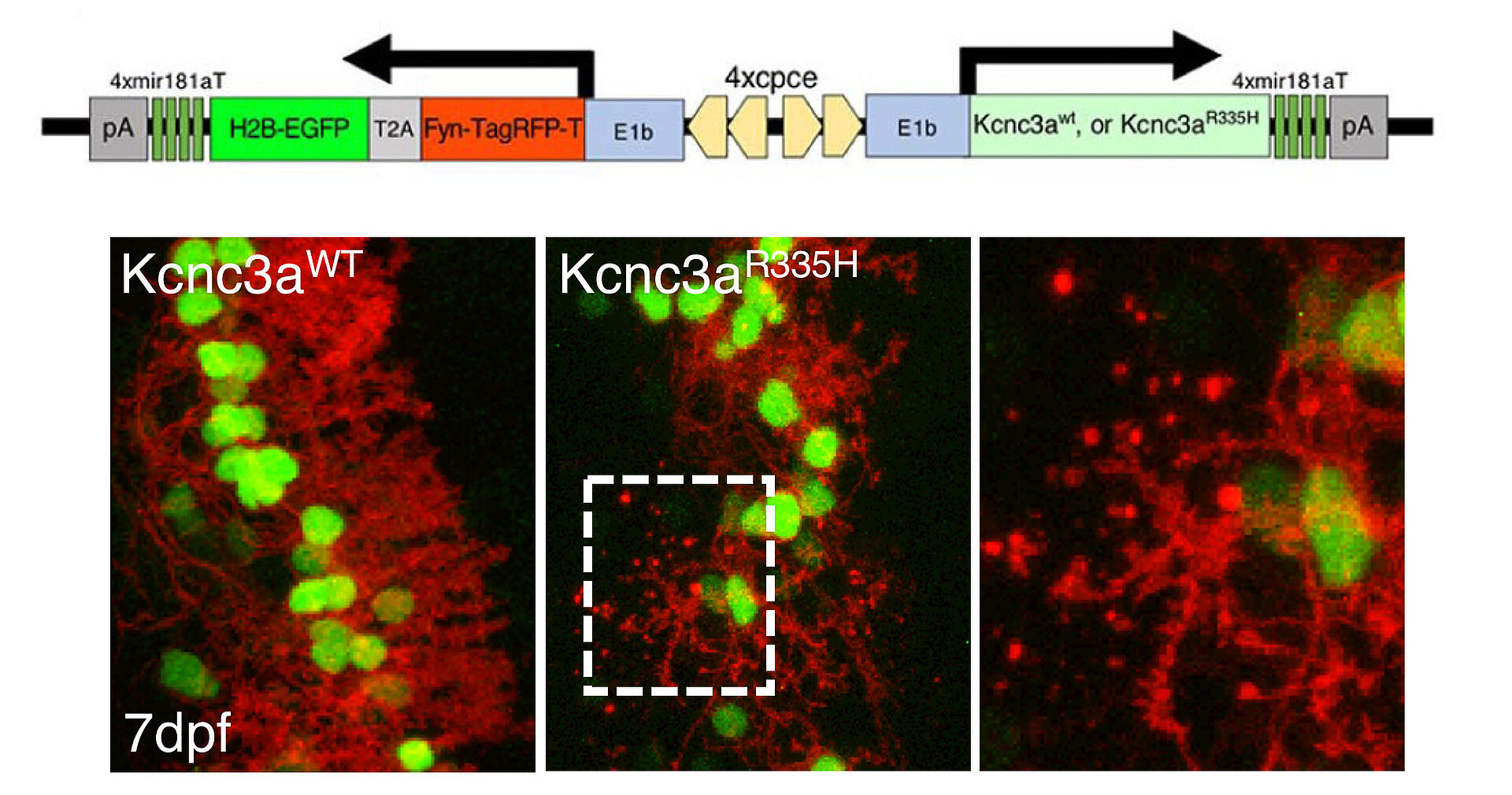
We already developed a cerebellar Purkinje neuron specific multi-color labeling method using a bidirectional expression system in transparent zebrafish (Fig. 1) in order to monitor pathophysiological processes occurring in degenerating neurons.
We have established transgenic zebrafish model mimicking SCA type 13 by overexpressing its causative mutant of KCNC3, a voltage gated potassium channel (Fig. 2, 3, and Movie).
Open positions
Positions for students to write bachelor/master thesis, or the internship course to acquire basic skills of molecular biology, culturing mammalian cells, or in vivo zebrafish imaging (at least for 3 months, or longer) are available. I would be also happy to assist you to write a grant proposal to support your stay in our group for PhD study/or Post-doc research.
Review articles describing our zebrafish models
Original articles related to the current research
VITA
Work experience:
Since 2013: Research fellow (permanent faculty position), Zoological Institute, Division of Cellular and Molecular Neurobiology, Technical University of Braunschweig, Germany
2011-2013: Research fellow, Zoological Institute, Department of Cell biology and Cell Physiology, Technical University of Braunschweig, Germany
2006-2010: Research fellow, Institute of Developmental Genetics, Zebrafish Neuroimaging Group, Helmholtz Center, Munich-Neuherberg, Germany
2005-2008: Associate professor, Department of Anatomy and Neuroscience, Asahikawa Medical University, Japan
2001-2005: Assistant professor, Department of Anatomy and Neurobiology, Osaka City University, Graduate School of Medicine Japan
1999-2001: Assistant professor, Department of Anatomy, Asahikawa Medical College, Japan
Education:
1995-1999: Ph.D. Study (Ph.D. degree in medicine, 2000, Osaka University, Japan)
1993-1995: Master Study (Master degree in Medical science, Osaka University, Japan)
1992-1993: Research student, Department of Developmental Medical Sciences, Division of Health Science, Faculty of Medicine, University of Tokyo, Japan
1990-1992: Undergraduate Study in Health Science, Division of Health Science, Faculty of Medicine, University of Tokyo, Japan
1998-1990: Undergraduate Study in Natural Sciences, University of Tokyo, Japan
Teaching:
Current teaching activities at Technical University of Braunschweig:
Past teaching experiences:
Publication list